Valve Disease
NewYork-Presbyterian offers pioneering programs in the treatment of valve disease, including interventional, surgical, and robotic options. Our clinicians across all cardiovascular specialties are at the vanguard of research and clinical trials in the development, evaluation, and refinement of innovative, less-invasive techniques for repairing and replacing aortic, mitral, and tricuspid valves.
Aortic Valve
Transcatheter aortic valve replacement has progressed dramatically in terms of device technologies and patient selection due, in large part, to the transformative work of interventional cardiologists and cardiac surgeons at NewYork-Presbyterian. Our physicians have served and continue to serve as principal investigators in landmark trials related to transcatheter valve therapies, including multicenter studies for aortic and mitral valve disease, and now tricuspid valve disease.

At NewYork-Presbyterian, recovery has been made easier for patients with a transition from the use of general anesthesia to conscious sedation or monitored anesthesia control. In fact, 75 percent of TAVR patients do not go to the ICU from the Catheterization Lab, but rather directly to an observation unit for recovery.
Partner 3
Having served as the principal investigators for the landmark trials that established TAVR as the standard of care for inoperable patients, as an alternative to surgery in high-risk patients, and most recently as another treatment option for patients at intermediate risk, Columbia and Weill Cornell faculty are now serving as principal investigators in a multicenter study in patients with symptomatic, severe aortic stenosis at low operative risk. The study is evaluating TAVR using the SAPIEN 3 balloon-expandable platform with surgical valve replacement in patients with low operative risk for symptomatic, severe aortic stenosis. This pivotal, groundbreaking trial, which has not been conducted anywhere else in the world, could potentially show that transcatheter valve replacement is no worse – or perhaps even better – than open surgical procedures.
Valve-in-Valve TAVR
Columbia and Weill Cornell clinician-researchers are also exploring TAVR as an option for patients with failing surgically implanted bioprosthetic valves. Rather than having a high-risk open chest procedure, selected patients may be candidates for having a new valve placed inside the older valve via TAVR. The early experiences of using valve-in-valve TAVR for structural degeneration of bioprosthetic surgical aortic valves and data from the PARTNER 2 Valve-in-Valve Registry in patients with symptomatic aortic stenosis at high risk for complications during a reoperation demonstrated that valve-in-valve TAVR presents a viable option for selected patients.
Early TAVR
NewYork-Presbyterian clinicians are also looking at two new categories of patients – those with moderate aortic stenosis in heart failure and those with severe asymptomatic aortic stenosis – with a goal to identify patients who may benefit from early TAVR before they present with atrial fibrillation, severe heart failure, or with pulmonary hypertension. To this end, researchers at Columbia and Weill Cornell are participating in major multicenter clinical trials with potentially significant implications for the aortic stenosis population.
- Early TAVR Trial Approved by the FDA in February 2017, this trial will compare the practice of active surveillance to early TAVR in patients diagnosed with severe aortic stenosis who have not yet developed symptoms.
- TAVR UNLOAD Trial Begun in May 2016, this international study is comparing TAVR performed via a transfemoral approach in combination with optimal heart failure therapy (OHFT) to OHFT alone in patients diagnosed with heart failure with reduced ejection fraction and moderate aortic stenosis. The investigators hypothesize that unloading the left ventricle by reducing the transaortic gradient with TAVR may improve the clinical outcomes of these patients.
Valve Procedures
Procedures by Valve Type
2016
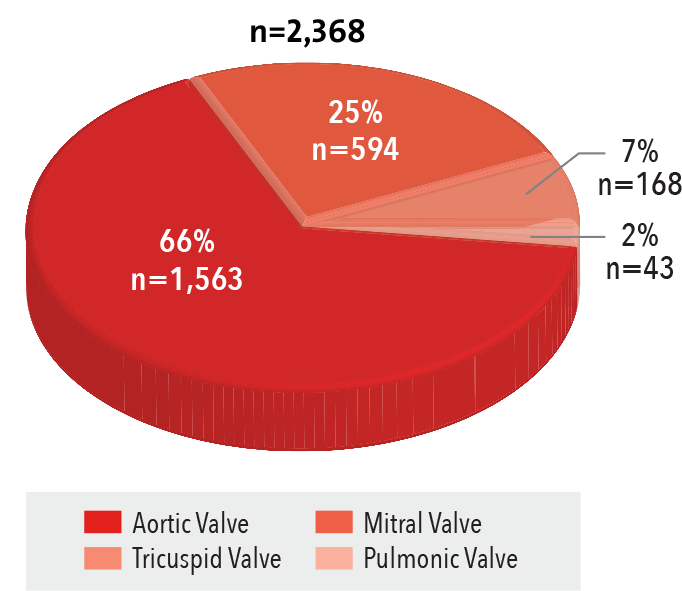
Source: NewYork-Presbyterian
Procedures by Approach
2016
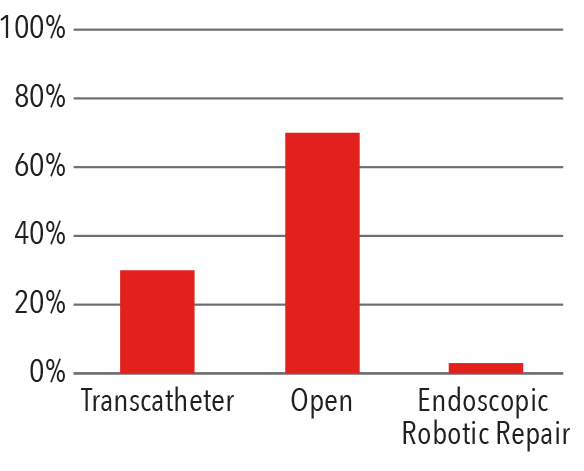
Source: NewYork-Presbyterian
Surgical Site Infection Rate
2012 - 2016
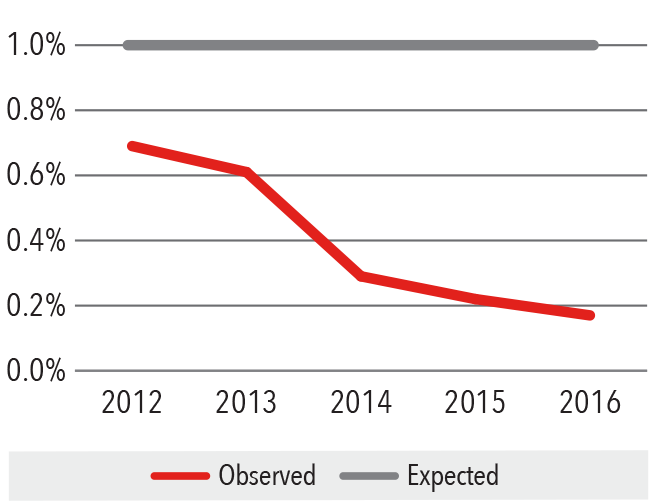
Standardized Infection Ratio = Observed/Expected
Source: National Healthcare Safety Network/
Department of Infection Prevention and Control
In-Hospital Mortality Rate
2016
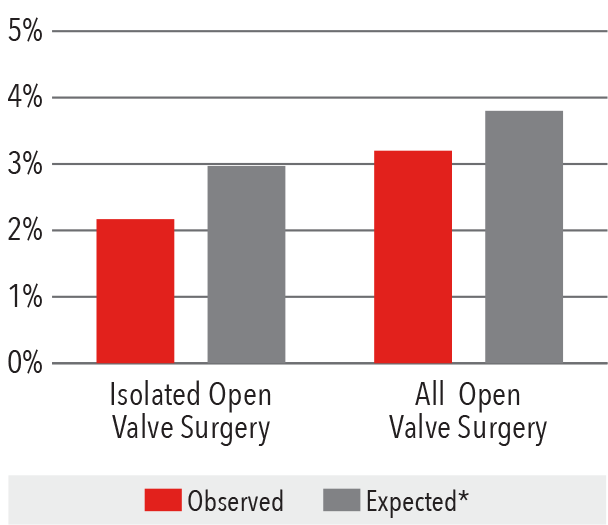
*Expected mortality was determined using
Vizient risk-adjustment methodology.
Source: Vizient Clinical Data Base/Resource ManagerTM
used by permission of Vizient. All rights reserved.
Transcatheter Valve Procedures
Volume
2012-2016
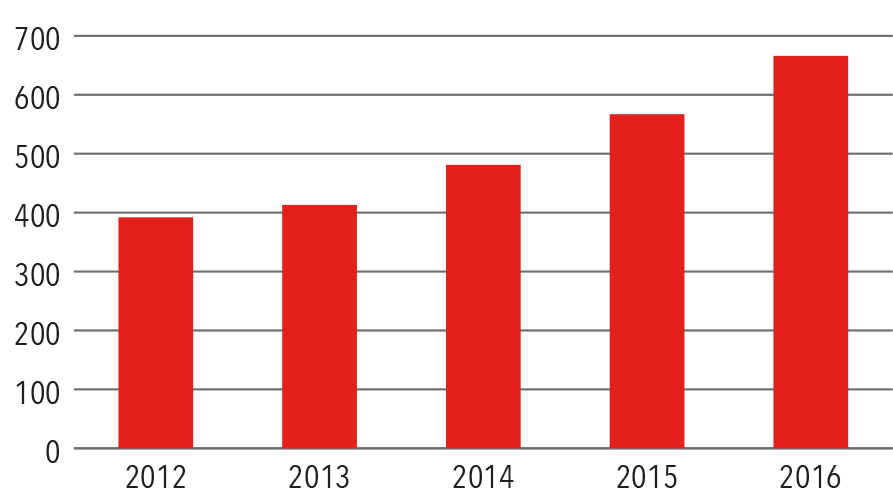
Source: NewYork-Presbyterian
Procedures by Access
2016
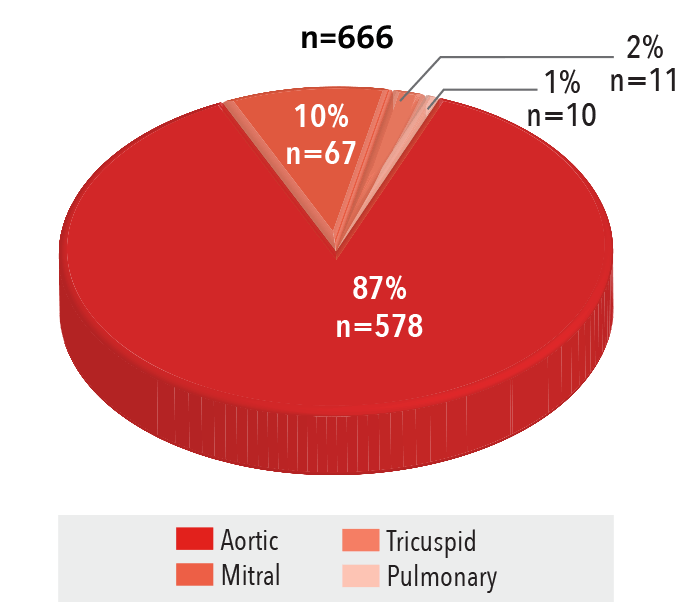
Source: NewYork-Presbyterian
In-Hospital Mortality Rate
2016
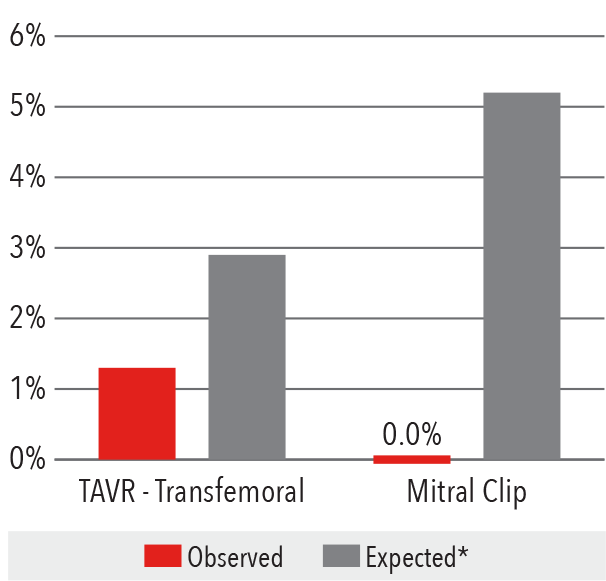
*Expected mortality was determined using
Vizient risk-adjustment methodology.
Source: Vizient Clinical Data Base/Resource ManagerTM
used by permission of Vizient. All rights reserved.
Aortic Valve Surgery
In-Hospital Mortality Rate
2016
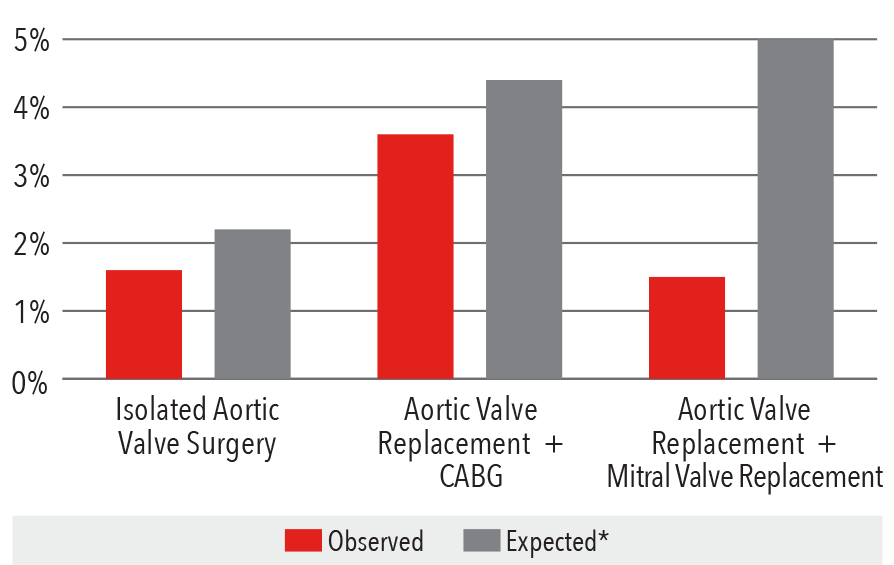
*Expected mortality was determined using Vizient risk-adjustment methodology
Source: Vizient Clinical Data Base/Resource ManagerTM
used by permission of Vizient. All rights reserved.
Isolated Aortic Valve Complications Rate, 2016

Source: Vizient Clinical Data Base/Resource ManagerTM
used by permission of Vizient. All rights reserved.
Mitral Valve
The majority of mitral valve surgery in the country is performed through full sternotomy; about 35 percent is done minimally invasively; and 5 percent is done robotically. At NewYork-Presbyterian, mitral valve replacement has been transitioning from full or partial sternotomy surgery to more minimally invasive keyhole, transcatheter, and robotic approaches.
Patients who come to NewYork-Presbyterian with mitral valve disease benefit from a comprehensive evaluation by a multidisciplinary heart team, which makes the determination of the appropriate therapeutic option based on several patient factors, including the severity of symptoms and comorbidities.
MitraClip

The MitraClip® NT Clip Delivery System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation. (Courtesy of Abbott)
Patients with significant symptomatic degenerative mitral regurgitation who are not candidates for surgery are benefitting from the FDA-approved MitraClip, a low-risk, catheter-based procedure that creates a tissue bridge between the anterior and posterior leaflets using one or two clips that are deployed through a transseptal approach. The MitraClip, which is now available for highrisk patients with degenerative mitral valve disease, continues under evaluation in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) multicenter trial.
Transcatheter Mitral Valve Replacement
For patients who are highly stenotic, MitraClip is not an option, and until recently, these patients would need to undergo an open surgical repair. Transcatheter mitral valve replacement (TMVR) is a new technology under study in feasibility clinical trials as a potential therapy for patients with symptomatic, severe mitral regurgitation. Performed through a small incision on the left side as in TAVR, TMVR involves a self-expanding, nitinol valve with bovine pericardial leaflets that is placed using a transapical delivery system. Columbia faculty were among the study’s principal investigators in centers throughout the United States, Australia, and Europe. Early experience with 50 patients has shown that the device implant is feasible in patients at high- or extreme-risk for conventional mitral valve replacement. The results are now guiding the trial design of TMVR in lowerrisk patients with severe mitral valve regurgitation.
Total Endoscopic Robotic Mitral Valve Repair
NewYork-Presbyterian’s cardiothoracic surgeons are now routinely performing endoscopic robotic surgery repair, particularly for patients with mitral valve regurgitation. The procedure is performed though four 8mm and one 12mm incisions – considered the smallest incisions used for this procedure in the nation. Advantages of this approach over traditional sternotomy, non-robotic, or robotic port access approaches is that rib spreading is not needed and approaching the valve robotically from the right side of the chest allows the valve and the heart to remain in their natural positions, rather than retracting the heart to expose the valve. A catheter-based system is used to place the patient on the heart-lung machine, which enables the surgery to be accomplished completely through the minute ports. The endoscopic approach also allows the surgeon to visualize the valve in its natural position, greatly facilitating the repair and resulting in a very high repair rate.
Mitral Valve Procedures
Open Surgery Volume Graph
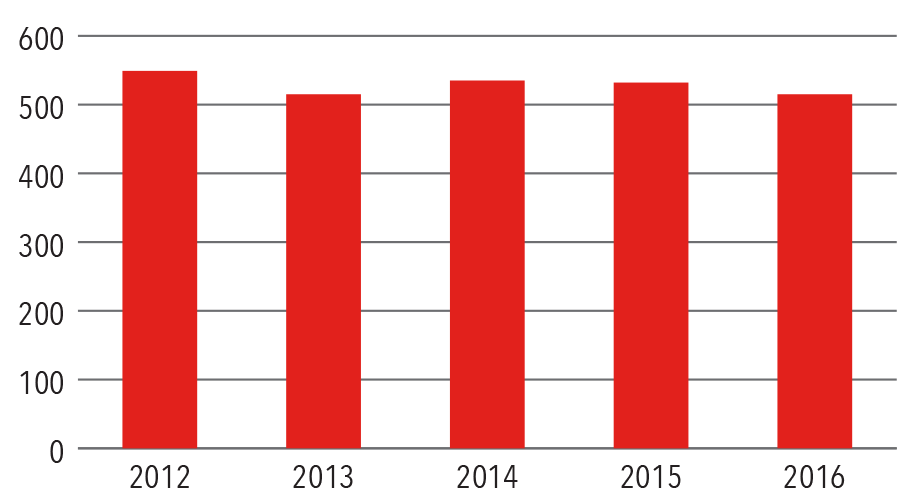
Source: NewYork-Presbyterian
Volume by Type
2016
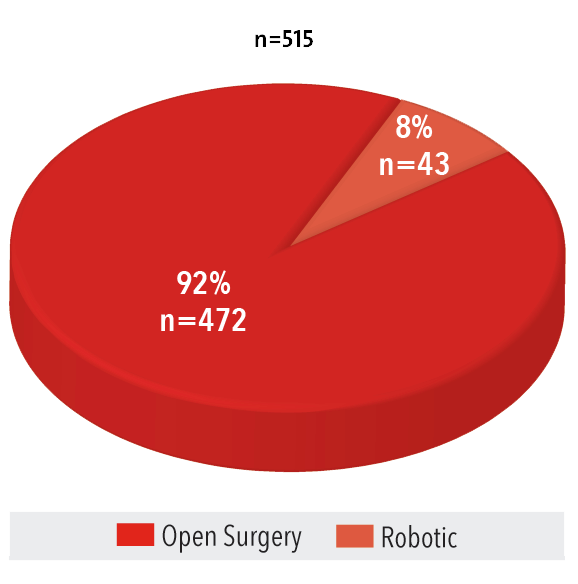
Source: NewYork-Presbyterian
In-Hospital Mortality Rate
2016
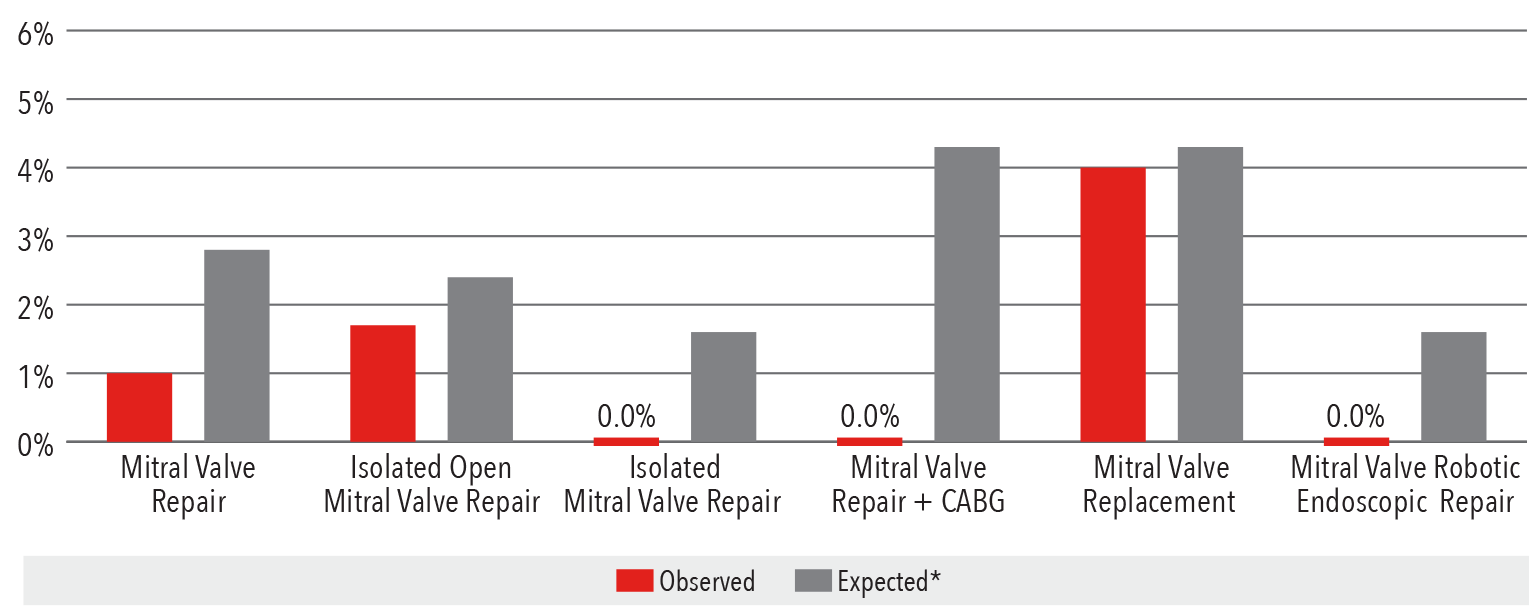
*Expected mortality was determined using Vizient risk-adjustment methodology.
**Isolated mitral valve includes both mitral valve repair and replacement.
Isolated Mitral Valve Complications Rate, 2016

Source: Vizient Clinical Data Base/Resource ManagerTM used by permission of Vizient. All rights reserved.
Tricuspid Valve
Treatment alternatives for patients with severe, symptomatic tricuspid regurgitation are limited, with medical therapy often ineffective and surgery associated with high operative mortality. Columbia is one of five centers in the United States that participated in an early feasibility study of the Edwards FORMA Tricuspid Transcatheter Repair System of patients with tricuspid valve regurgitation. Clinical outcomes at 30 days showed that there was significant reduction of tricuspid regurgitation, especially in those patients with the worst baseline, and, at 30 days, there was significant improvement in NYHA functional class, six-minute walk tests, and KCCQ scores. Longer-term follow-up is necessary to assess recurrence, evidence of right ventricular remodeling, and late clinical outcomes.



