Immunotherapy: A New Frontier in Genitourinary Cancers

Dr. Charles G. Drake
In November 2016, Charles G. Drake, MD, PhD, joined NewYork-Presbyterian/Columbia University Medical Center as the new Director of Genitourinary Oncology. He also serves as Co-Director of Columbia’s Cancer Immunotherapy program along with Naiyer Rizvi, MD, Director of Thoracic Oncology at Columbia. “I was presented with a unique opportunity to build a genitourinary cancer group with the focus on trying to cure these cancers with the immune system, and doing so among a group of clinicians and scientists who, like Dr. Rizvi, are tops in the immunotherapy world.”
Dr. Drake emphasizes that his attraction to joining Columbia is the leadership of Stephen G. Emerson, MD, PhD, Director, and Gary K. Schwartz, MD, Deputy Director, of the NCI-designated Herbert Irving Comprehensive Cancer Center, where Dr. Drake also now serves as Associate Director for Clinical Research. “They have been shaping a progressive and comprehensive cancer program in which one of the cornerstones of their vision is the conviction that the immune system can produce meaningful and long-lasting clinical responses in cancer patients. This is what I’ve believed in for a long time and have focused on in my work for nearly two decades.”
A physician-scientist, Dr. Drake has established a career that encompasses both the laboratory and the clinic. His work spans the continuum of research from seeking understanding of basic immunological mechanisms to using animal models to understand how drugs work on certain molecules to evaluating immuno-based therapies in clinical trials.
After receiving a B.S. in engineering from Rutgers University College of Engineering and a master’s degree in bioengineering at Rutgers, Dr. Drake then pursued his PhD in immunology at the National Jewish Center for Immunology and Respiratory Medicine/University of Colorado Health Sciences Center. Two years later, he completed his MD there. A residency and medical oncology fellowship followed at Johns Hopkins University, where Dr. Drake joined the faculty, rising to become Professor in Oncology, Urology, and Immunology and Co-Director of the Cancer Immunology Program, while continuing incisive research in immunology that had begun during his graduate studies. In more than 80 articles and book chapters, and with many inventions and patents, Dr. Drake has advanced the use of immunotherapies in combination with existing treatments, both surgical and nonsurgical, and across tumor types, including those at advanced stages.
Extending the Boundaries of Immunotherapy
“Immunotherapy represents a new frontier in genitourinary cancers,” says Dr. Drake. “We’re looking at how it can be used synergistically with traditional therapies in prostate cancer as well as in other tumor types.”
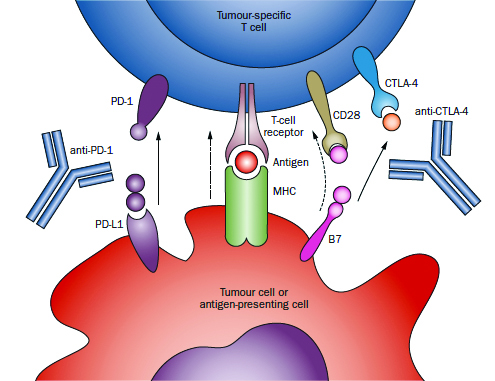
Immune checkpoint blockade approach to immunotherapy is exemplified by antibodies directed against CTLA-4 (ipilimumab, tremilimumab), which block the immunosupression mediated by the interaction between B7 family members (on antigen-presenting cells) and CTLA-4 (on CD8+ and CD4+ T cells). A second major checkpoint, mediated by the interaction between PD-1 on T cells and its ligand PD-L1 on either antigen-presenting cells or tumour cells, has been the subject of several clinical trials, showing evidence efficacy in both non-small-cell lung cancer and renal cell carcinoma.
Source: Nature Reviews | Clinical Oncology. January 2014.
Click the image above to view larger image.
Revealing a promising and growing understanding of the mechanisms that encourage the immune system to ignore malignant invaders, Dr. Drake has been a leading voice for the efficacy of immunotherapy. He has been a lead author or integral participant in lauded research and review articles that underscore immunotherapy’s re-emerging potential. These include “Prostate Cancer as a Model for Tumor Immunotherapy,” published in Nature Reviews Immunology; “Breathing New Life Into Immunotherapy: Review of Melanoma, Lung and Kidney Cancer,” published in Nature Reviews Clinical Oncology; “New Strategies in Bladder Cancer: A Second Coming for Immunotherapy,” published in Clinical Cancer Research; and “Recent Advances in Immunotherapy for Kidney Cancer,” published in Discovery Medicine.
Early on in his career at Johns Hopkins, Dr. Drake developed a novel transgenic model of prostate cancer in which a unique antigen is expressed exclusively in the prostate gland and in prostate tumors. That model made it possible for researchers to validate that androgen ablation, the most common treatment for progressive prostate cancer, can break tolerance to the prostate gland, lending itself to anti-tumor vaccination. “My team worked very hard to gather immune cells that are infiltrating different kinds of genitourinary cancers,” says Dr. Drake. “We’ve gotten immune cells from prostate, kidney, and bladder cancers, and we’ve studied them very, very carefully using a technique called RNA-Seq that tells us about every single gene and how it’s expressed, and actually how it’s spliced, too. It’s absolutely fascinating.”
His research group also discovered several new molecules that made it into the clinic. “The one that is the farthest along is a molecule called LAG-3 [lymphocyte activation gene-3],” notes Dr. Drake. “We showed that LAG-3 could restore anti-tumor immune responses and that blocking multiple immune checkpoints might be required to restore anti-tumor immunity. This molecule is now in Phase I trials in multiple tumor types.”
Dr. Drake notes that for a long time it was thought that patients diagnosed with different genitourinary cancers, particularly prostate and kidney cancers, should be rushed into surgery. For example, in patients with high-grade kidney cancer, surgery will cure many of those patients, 60 to 70 percent, but not all. “Many studies have now shown that delaying surgery four to six weeks, particularly if patients are undergoing active treatment, is not clinically significant.”
In a recent trial initiated by Dr. Drake at Hopkins, selected patients determined to be at a high risk for a return of the cancer following surgery were given three doses of the immunotherapy drug anti-PD-1 two weeks apart prior to undergoing surgery. “The beauty of this from the patients’ point of view is that this treatment approach may reduce the chances or recurrence,” says Dr. Drake. “This is also, of course, a plus for clinicians, but additionally, at the time of surgery we have the entire kidney tumor available for testing. By analyzing that specimen carefully, we can get a good idea of what a particular immune drug does or doesn’t do. Does it fix the CD8 cells or regulatory T cells? Does the therapy address other populations of immune cells that might be important? From a scientific standpoint, there’s no better way to understand what these drugs do in patients in general, but also, tellingly, in particular patients. We’re learning a lot already from that small trial.”
In a trial with prostate cancer, Dr. Drake and his team tested hormonal therapy versus hormonal therapy and a vaccine. “The vaccine has been around for a long time,” Dr. Drake explains, “and it was fascinating to see that the hormonal therapy did a nice job of driving activated CD8 cells into the prostate gland. It was statistically significant, it was tolerated, and it was a really fascinating result. We’re still analyzing all the tissue, but are finding that the vaccine didn’t really add all that much. By doing these neoadjuvant trials, we can accelerate the development of new agents or new combinations of agents. Once we have a good signal in this early stage, we can take the agents to the later stages to look for more obvious signs of clinical activity or hopefully clinical benefit.”
At Columbia, Dr. Drake continues his groundbreaking research and is excited about drawing on the extensive expertise and progressive research underway here by cancer specialists and molecular biologists across disciplines. “We want to establish collaborative tumor specific working groups in prostate, kidney, and bladder cancers, with a particular focus on crafting a clinical trials portfolio focused on later stage diseases,” says Dr. Drake. “The goal is to have cutting-edge clinical trials across the diseases and then across the spectrum of diseases. From the very beginning, we want to develop trials where in addition to standard radiation or surgery, we give patients a drug to activate the immune system so that the conventional treatment is more likely to lead to a long-term response without the cancer coming back.”
Reference Articles
Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. Journal of Clinical Oncology. 2017 Jan;35(1):40-47.
McNeel DG, Bander NH, Beer TM, Drake CG, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of prostate carcinoma. Journal for Immunotherapy for Cancer. 2016 Dec 20;4:92.
Danilova L, Wang H, Sunshine J, Kaunitz GJ, Cottrell TR, Xu H, Esandrio J, Anders RA, Cope L, Pardoll DM, Drake CG, Taube JM. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proceedings of the National Academy of Sciences USA. 2016 Nov 29;113(48):E7769-E7777.
Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clinical Cancer Research. 2016 Nov 15;22(22):5461-71.
Drake CG, Bivalacqua TJ, Hahn NM. Programmed cell death ligand-1 blockade in urothelial bladder cancer: To select or not to select. Journal of Clinical Oncology. 2016 Sep 10;34(26):3115-16.
Ball MW, Allaf ME, Drake CG. Recent advances in immunotherapy for kidney cancer. Discovery Medicine. 2016 Apr;21(116):305-13.
Mellman I, Hubbard-Lucey VM, Tontonoz MJ, Kalos MD, Chen DS, Allison JP, Drake CG, et al. De-risking immunotherapy: Report of a Consensus Workshop of the Cancer Immunotherapy Consortium of the Cancer Research Institute. Cancer Immunology Research. 2016 Apr;4(4):279-88.
Alme AK, Karir BS, Faltas BM, Drake CG. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: An overview. Urologic Oncology. 2016 Apr;34(4):171-81.
Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nature Reviews. Clinical Oncology. 2014 Jan;11(1):24-37.
Related Publications





