Faculty at the NCI-designated Herbert Irving Comprehensive Cancer Center at Columbia University and the Weill Cornell Medicine Meyer Cancer Center in partnership with NewYork-Presbyterian are pioneering innovative strategies for early detection and optimal diagnosis and accelerating the discovery and development of novel therapeutics to achieve the best possible outcomes in cancer patients. Additionally, physicians and researchers work to obtain FDA approval of drugs that combat cancer. Taken together, the progress made by Columbia and Weill Cornell researchers and physicians is having a large impact upon cancer diagnosis, therapy, and prognosis.
Harnessing New Therapies for Pancreatic Cancer
Applying Organoid Models to Accelerate Cancer Care
Bringing Tumor Cells Out of the Shadows
Radiation Therapy Delivered in a FLASH

Harnessing New Therapies for Pancreatic Cancer
Columbia and Weill Cornell clinicians and researchers are seeking to upend the prognosis for pancreatic cancer.
Recently, Columbia researchers conducted an exciting study that suggests a compound that starves tumors of the amino acid cysteine, critical to the survival of pancreatic cancer cells, may have potential in the fight against this deadly disease.
Weill Cornell investigators are co-leading an innovative international phase 2a clinical trial testing a triple combination of an investigational drug that enables immune cells to infiltrate tumors, an immunotherapy drug, and chemotherapy that has shown promising responses in patients with metastatic pancreatic cancer.
Light micrograph of a section showing desmoplasia associated with pancreatic cancer

Applying Organoid Models to Accelerate Cancer Care
Columbia and Weill Cornell scientists continue to pursue organoid research to accelerate the pace of drug screening to identify more precise treatment options for patients. At Weill Cornell, researchers are developing vascularized organoids with a goal to produce functional blood vessels as a way to bring nutrients or immune cells into a tumor. The result would be cultures that are near perfect copies of a patient’s tumor theorizing that any therapy that works in those tumor cells is also likely to work in the patient. At Columbia, scientists are applying organoids as a suitable model system for studying tumor evolution, mechanisms involved in treatment response, and the ability of organoids to predict responses to therapy prospectively, with a focus on bladder and GI (esophageal, pancreatic, and colon) cancers. Many of these studies are now possible because of the capability of organoids to model tumor heterogeneity.
Organoids created from the bladder cancers of patients mimic the characteristics of each patient’s tumor. (Courtesy of Dr. Michael Shen)

Bringing Tumor Cells Out of the Shadows
According to new preclinical research by Weill Cornell investigators, localized radiation therapy against a tumor can trigger a beneficial immune response throughout the body by releasing DNA from mitochondria into the cytoplasm of tumor cells. Having previously determined that autophagy was helping to protect the tumor cells from immune detection, the scientists further identified that this DNA was coming from the cells' mitochondria. These findings taken together point to a model in which radiation favors the accumulation of mitochondrial DNA outside of mitochondria in tumor cells, but autophagy allows the cells to hide those fragments from the immune system and prevent the abscopal response. Exploring the use of mitochondria-targeting drugs to exploit this novel mechanism and improve patients’ responses to radiation therapy is an area with great translational potential.
Irradiated breast cancer cells release DNA (red) from mitochondria (green), setting off an alarm for the immune system. The blue color marks nuclei.
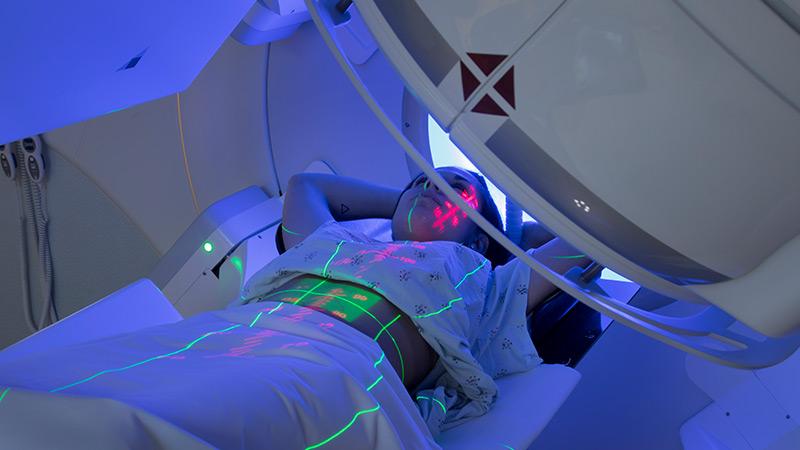
Radiation Therapy Delivered in a FLASH
A team of Columbia radiation oncologists, radiation biologists, and radiation physicists is studying the emerging technique of FLASH radiotherapy, the delivery of ultra-high-dose radiation a thousand times quicker than conventional therapy. Reducing the treatment time to a fraction of a second can decrease the detrimental effects to normal tissue near the tumor without sacrificing the effectiveness of the radiotherapy. The Columbia team is exploring in in vivo mice studies whether FLASH radiation treatment can lead to an even greater immune response in patients with cancer than with conventional radiation. They are also examining the role oxygen depletion plays in FLASH’s ability to kill targeted tumors while sparing adverse effects on surrounding healthy tissue.
Extending Survival in Esophageal Cancer
A study by Weill Cornell researchers has shown that the immunotherapy drug pembrolizumab is a safe and effective option for patients with locally advanced and metastatic esophageal squamous cell cancer who have already received standard chemotherapy. The study reports on the results of a phase 3 multicenter clinical trial led by Weill Cornell in which the drug extended overall survival by more than two months in a subgroup of patients, providing another treatment option for patients who typically have a poor prognosis overall. The trial findings contributed to the FDA’s approval of pembrolizumab as a second-line treatment for patients with esophageal squamous cell cancer who also have high levels of PD-L1, the target of the drug.
3D illustration of a molecular model of pembrolizumab, which targets the PD-L1 receptor of lymphocytes and is used in cancer immunotherapy.

Staying Ahead of Stomach Cancer
A new study by Columbia researchers suggests that immunotherapy for stomach cancer may work better if delivered earlier in the course of disease concurrently with standard chemotherapy. They discovered that mice with more advanced disease had an abundance of myeloid-derived suppressor cells (MDSCs) that also express PD-L1 proteins, which appear to overpower the immunotherapy. When immunotherapy was given to mice with these advanced tumors, the cancer was unaffected. Only when immunotherapy was given to the mice before tumors formed and prior to the accumulation of MDSCs could cancer progression be slowed, indicating that adding chemotherapy to immunotherapy may improve responsiveness in part through the targeting of MDSCs.
Stomach cancer cells from a mouse model; PD-L1 molecules are stained pink.



