Advanced Heart Failure
Noteworthy Progress in Heartmate 3™
On August 28, 2018, the FDA approved the HeartMate 3™ left ventricular assist device (LVAD) as a destination therapy for patients with advanced heart failure who are not eligible for transplant, as a life-long implant. The HeartMate 3™ LVAD was approved as bridge to transplant and as a bridge to myocardial recovery in a preceding year. FDA approval was supported by the landmark MOMENTUM 3 Trial — the largest randomized trial in the history of mechanical circulatory support — which included >1,000 patients. New York-Presbyterian/Columbia surgeons and cardiologists were at the forefront of this innovation: They were among the first to offer patients access to HeartMate 3™ nationwide, enrolled the largest number of patients (>6 percent of the entire enrollment), and commemorated entry of the 100th patient into the trial.
The device is available to patients at both NewYork-Presbyterian/Columbia and NewYork-Presbyterian/Weill Cornell.
The HeartMate 3™, a fully magnetically levitated centrifugal continuous-flow pump, markedly reduced complications over its predecessors, including:
-
lowest published stroke rate (3-fold reduction ≥6 month post implant)
-
eliminated pump thrombosis, one of the major complications of device therapy
Overall, LVAD therapy has now reached unprecedented success, as evidenced by:
-
survival of 83 percent at two years, which is now comparable to heart transplantation
-
increased exercise capacity — doubled six-minute walk distance (from 154 to 308 meters)
-
improved quality of life based on the patient responses to Kansas City Cardiomyopathy and European Quality of Life Questionnaires

NewYork-Presbyterian is one of 16 programs in the world to be designated as a Platinum Center of Excellence in Life Support by the Extracorporeal Life Support Organization.
Adult ECMO
Volume
2013-2017

Source: NewYork-Presbyterian
Type of Procedure
2017

Source: NewYork-Presbyterian
NewYork-Presbyterian Heart Transplant Program in the Era of the New Organ Allocation System
This past year also brought significant changes to the National Heart Allocation Policy, which now allows for 500 mile broader sharing of organs for the sickest patients and earlier access to transplant for specialized patient populations, including adult patients with congenital heart disease (CHD) and hypertrophic cardiomyopathy. NewYork-Presbyterian/Columbia physician leadership took on a national role in this policy development and implementation, which has already shown to increase heart transplantation opportunities in New York State.
Other program highlights include:
-
growth in our multi-organ transplant program, including heart-kidney and heart-liver transplantation, the latter also for patients with mutational TTR amyloidosis and for adult CHD patients with Fontan-associated liver disease
-
a new program for the use of hepatitis C viremic donors to increase heart transplantation opportunities for those patients with longer waiting time on the list; a course of anti-viral therapy is administered at 12 weeks after transplant to cure the transmitted hepatitis C virus
-
initiation of a protocol to offer novel immunosuppressive treatment with Belatacept to desensitize patients and thereby increase their heart transplantation opportunities
Ventricular Assist Device Implants
Volume
2013-2017

Source: NewYork-Presbyterian
Volume Distribution
2017
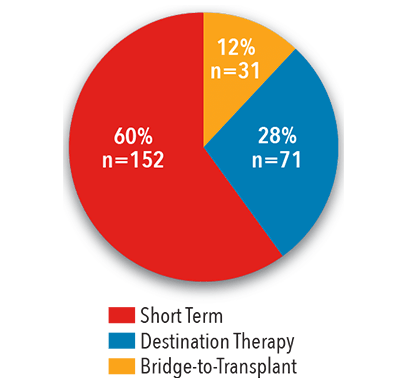
Source: NewYork-Presbyterian
In-Hospital Mortality
2017
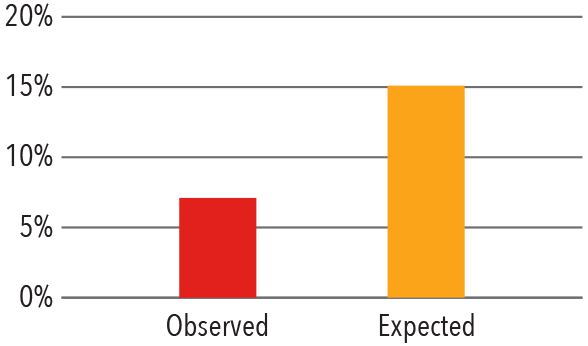
Source: Vizient Clinical Data Base/Resource Manager™ used
by permission of Vizient. All rights reserved.
Patient Profiles INTERMACS*
June 23, 2006 - December 31, 2017

*INTERMACS is the United States National Registry for patients receiving durable mechanical circulatory
support device therapy to treat advanced heart failure.
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2017)
Adverse Events*
June 23, 2006 - December 31, 2017
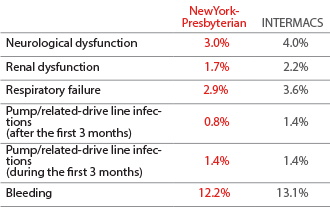
*Table includes overall counts and percentages for each type of
adverse event reported at Hospital site and INTERMACS overall.
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2017)
All Long-Term Implants Post-Implant Survival
June 23, 2006 - December 31, 2017

Source: INTERMACS Quality Assurance Quarterly Report (Q4 2017)
Bridge-to-Transplant Survival
June 23, 2006 - December 31, 2017

Source: INTERMACS Quality Assurance Quarterly Report (Q4 2017)
Destination Therapy Survival
June 23, 2006 - December 31, 2017
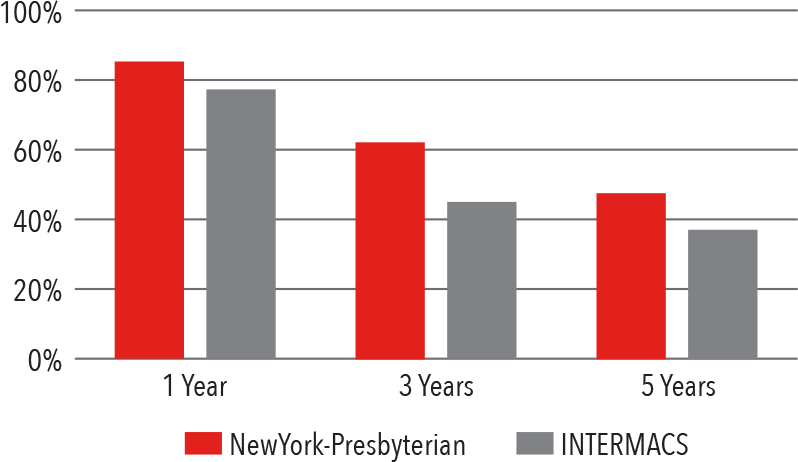
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2017)
NewYork-Presbyterian has performed 2,561 heart transplants between January 1988 and June 2018 — the largest volume in New York State — and maintains the largest waiting list in the state.
Adult Heart Transplant
Survival
2012 - 2017
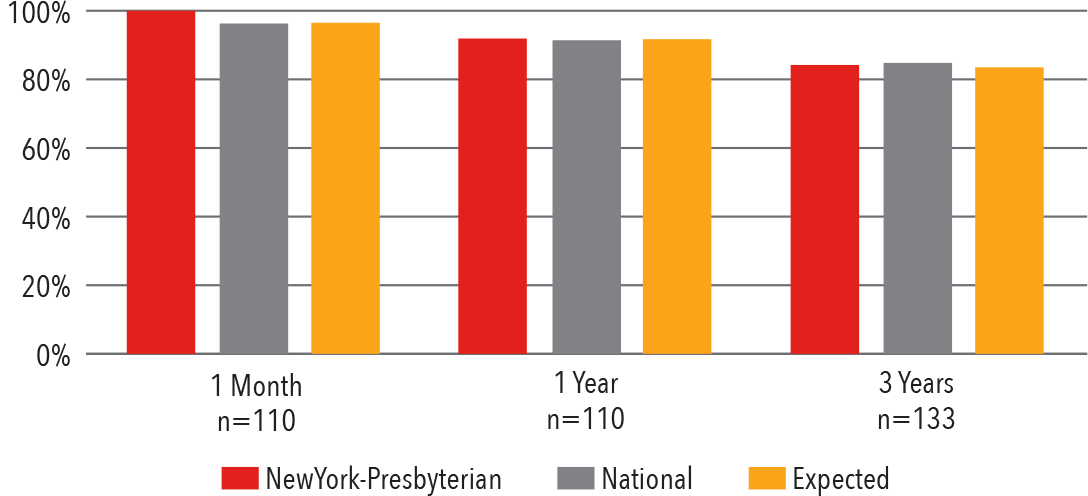
1 month (adjusted) January 1, 2015 - June 30, 2017 1 year (adjusted) January 1, 2015 - June 30, 2017 3 years (adjusted) July 1, 2012 - December 31, 2014 Source: Scientific Registry of Transplant Recipients / srtr.org Release Date: July 26, 2018
In-Hospital Mortality
2015 - 2017
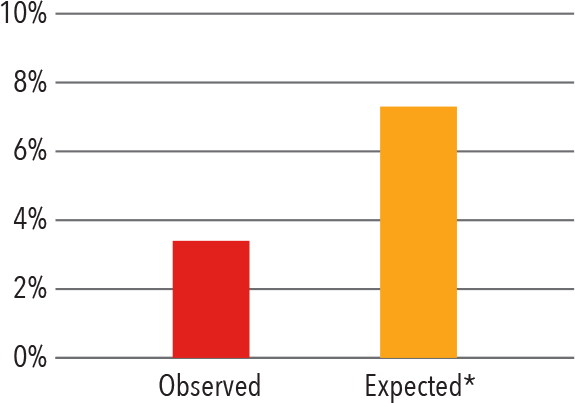
*Expected mortality was determined using Vizient risk-adjustment methodology. Source: Vizient Clinical Data Base/Resource Manager™ used by permission of Vizient. All rights reserved.
Advanced Heart Failure
Mortality
July 1, 2014 - June 30, 2017
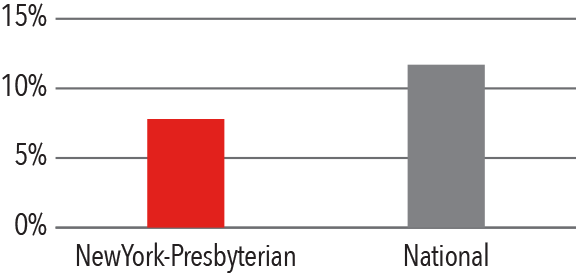
Source: Centers for Medicaid and Medicare Services/medicare.gov/hospitalcompare
Heart Failure with Preserved Ejection Fraction
Researchers in the Greenberg Division of Cardiology at Weill Cornell undertook a study using the National Inpatient Sample to better characterize differences in management and in-hospital outcomes of atrial fibrillation among patients with heart failure with preserved ejection fraction (HFpEF) and those with heart failure with reduced ejection fraction (HFrEF). They found that the prevalence and adverse impact of atrial fibrillation on those with HFpEF was substantial, providing a rationale to rigorously investigate strategies, such as rhythm control, to improve outcomes for this vulnerable subpopulation.
Weill Cornell recently started a dedicated HFpEF program, which combines expertise in heart failure and geriatric cardiology. In addition to providing state-of-the-art clinical care, the HFpEF program also serves as a platform for several ongoing research studies sponsored by both the NIH and industry. This research is focused on multiple domains, including PH-HFpEF through our NIH PVDOMICS study and clinical trials; geriatric cardiology through a growing registry that characterizes the functional and cognitive impairments observed in HFpEF; and precision medicine through a collaboration with the Englander Institute for Precision Medicine at Weill Cornell.
Contending with Cardiogenic Shock
Columbia faculty have developed a novel minimally invasive surgical approach that combines extracorporeal membrane oxygenation (ECMO) and the external temporary ventricular assist device (Ec-VAD) for cardiogenic shock as a shortterm circulatory support. Comparing the outcomes to conventional external biventricular assist device support, patients who received Ec-VAD required fewer transfusions, had a significantly lower incidence of major bleeding events during support, and a greater percent survived to the next destination and had better overall one-year survival rates.
In a review of 124 patients who received ECMO or short-term surgical VAD following acute myocardial infarction, researchers at Columbia found that of the 55.6 percent who survived to discharge, 37.7 percent required heart replacement therapy and 62.3 percent were discharged without heart replacement therapy. Age and cardiac index at the time of implantation were predictors of survival to discharge. In another study to determine predictors of survival for patients with acute decompensated heart failure requiring venoarterial ECMO, Columbia researchers found that markers of chronic disease were powerful predictors of outcomes, while markers of clinical acuity, including hemodynamics, vasopressor/inotrope use, and lactate are not. The vast majority of survivors required durable left ventricular assist devices.
Novel Therapeutics
Transthyretin Cardiac Amyloidosis: Overlooked and Treatable
Transthyretin cardiac amyloidosis was thought to be a rare clinical condition, but according to Columbia cardiologists, the disorder has been underappreciated. In cardiac amyloidosis, the protein transthyretin dissociates and monomers misfolds and aggregate predominantly in the heart, leading to a cardiomyopathy and progressive heart failure. A diagnosis previously required extensive testing, including an endomyocardial biopsy, and even with a diagnosis, a successful treatment did not exist. With several discoveries by Columbia faculty, cardiac amyloidosis is now easier to diagnose and is treatable.
The researchers not only identified that a noninvasive scan using the isotope technetium pyrophosphate, when coupled with blood tests, negates the need for a biopsy, they tested a compound that dramatically reduces morbidity and mortality. A phase 3 clinical trial co-chaired by Columbia faculty has shown that the drug tafamidis in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) reduced deaths by 33 percent, reduced cardiovascular-related hospitalizations by 32 percent, and slowed the decline in quality of life among the 441 patients enrolled in the 2 ½-year study. Results of the study, published in the January 10, 2019, issue of The New England Journal of Medicine, found that the drug also slowed decline in functional capacity and quality of life and was generally well tolerated. If tafamidis receives FDA approval for transthyretin amyloid cardiomyopathy, it would be the first medical therapy for this life-threatening disease.



