Advanced Heart Failure
NewYork-Presbyterian’s advanced heart failure programs offer a continuum of care for patients in all stages of heart failure – from medical management to mechanical circulatory support and heart transplantation. At the core of these programs are multidisciplinary teams with highly specialized expertise in assessment and management of patients with challenging comorbidities.
Advanced Heart Failure
Mortality Rate
July 1, 2013 - June 30, 2016
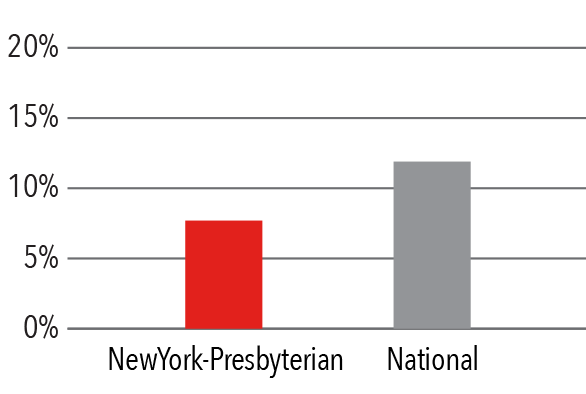
Source: Centers for Medicaid and Medicare Services /
medicare.gov/hospitalcompare
Most recently, new programs have been established dedicated to the care of patients with diastolic heart failure and a second program focused on the elderly population with heart failure. With an emphasis on preventing hospital admissions and reducing emergency room visits, our physicians have been utilizing the CardioMEMSTM device for continuous monitoring of pulmonary pressure. The FDA-approved heart failure monitoring system facilitates early recognition of fluid accumulation by enabling patients to transmit daily pressure trend information and pulmonary artery pressure waveforms for immediate physician review.
Preeminence in ECMO
Transferring Patients Needing ECMO
As the Hospital’s ECMO program has grown, our ECMO teams have continually refined protocols for safely transporting extremely ill patients from other hospitals who need this highly specialized care. A recent issue of The Annals of Thoracic Surgery highlighted our ECMO program, which conducted a review of 222 patients transported to NewYork-Presbyterian while supported with ECMO to evaluate survival during a surge period. A surge is defined as at least eight transports during a one-month period or patients transported within 24 hours of another patient in nonsurge months. The key message for achieving optimal outcomes for ECMO transport patients is to have welldefined interhospital communication processes, patient selection criteria, and management protocols that include consideration of staff fatigue and burnout for handling transient increases in volume.
ECMO and Pregnancy
ECMO is being used with increasing frequency to support pregnant and postpartum patients with severe cardiac or pulmonary failure. A recent study undertaken by Columbia clinicians in cardiothoracic surgery, pulmonary and critical care, maternal-fetal medicine, and pediatric cardiology described the largest case series of patients treated with ECMO during pregnancy and postpartum. Published in The Annals of Thoracic Surgery, the study results supported the use of ECMO during and following pregnancy, with favorable maternal and fetal outcomes that outweigh the risk of bleeding or thrombotic complications when managed by an experienced, multidisciplinary team.
Adult ECMO
Volume
2012-2016
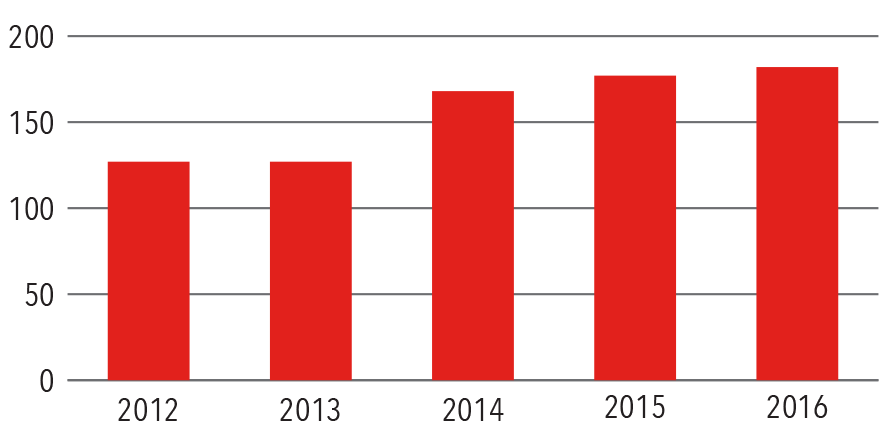
Source: NewYork-Presbyterian
Adult ECMO by Type
2016

Source: NewYork-Presbyterian
New Developments in Ventricular Assist Devices
NewYork-Presbyterian’s cardiovascular teams are highly experienced in the field of ventricular assist devices. Clinical investigations by faculty at Columbia and Weill Cornell encompass virtually every aspect of these devices, from their design to implantation techniques to the multiple factors that can affect outcomes.
Momentum 3 Trial
Columbia faculty were major participants in the Momentum 3 trial, the largest left ventricular assist device (LVAD) trial to date, and are now involoved in the continuous access protocol component. The trial evaluated HeartMate 3, which is smaller and more easily implantable than previous devices, but also features a centrifugal-flow system that utilizes fully magnetically levitated technology engineered to lower adverse event rates, especially thrombosis. The results, which were published in the February 2, 2017 issue of The New England Journal of Medicine, showed that this pump design was associated with better outcomes at six months when compared to the earlier axial-flow pump device, primarily because of the lower rate of reoperation for pump malfunction. In August 2017, the FDA approved the HeartMate 3 Left Ventricular Assist System as a bridge-to-transplant and this latest device is now being widely used by our cardiac surgeons.
Short-Term Mechanical Circulatory Support
Predicting survival of patients with cardiogenic shock following a heart attack and weaning them from short-term mechanical circulatory support devices remains largely unknown. Columbia physicians recently completed a study on 124 patients who received ECMO or VAD therapy at NewYork-Presbyterian finding that age and cardiac index were predictors for survival to discharge and recovery without durable VAD or transplant. While angiographic result and cardiac index predict ventricular recovery, 50 percent of patients optimally revascularized still required heart replacement therapy.
Highlights in Heart Transplantation
Transplant Outcomes After Device Implantation
Recent research undertaken by Columbia faculty focused on outcomes of heart transplantation in patients who had a mechanical circulatory device implanted as a bridge-to-transplant. Comparing short- and long-term outcomes in more than 400 patients, the researchers concluded that using various mechanical circulatory support devices can provide comparable clinical outcomes to primary heart transplant.
National Donor Heart Selection Conference
NewYork-Presbyterian was one of 12 heart transplant centers participating in authoring the report of the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States published in the October 2017 issue of the American Journal of Transplantation. The conference resulted in established agreement on the most important donor and recipient risk factors for donor selection and identified the components necessary for a future donor risk score.
Ventricular Assist Devices
Volume
2012-2016
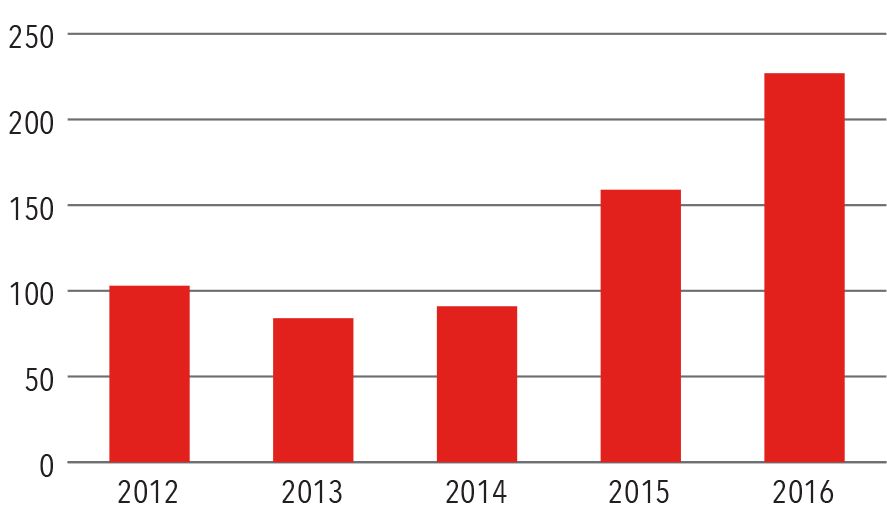
Source: NewYork-Presbyterian
Patient Profiles INTERMACS*
June 23, 2006 - December 31, 2016
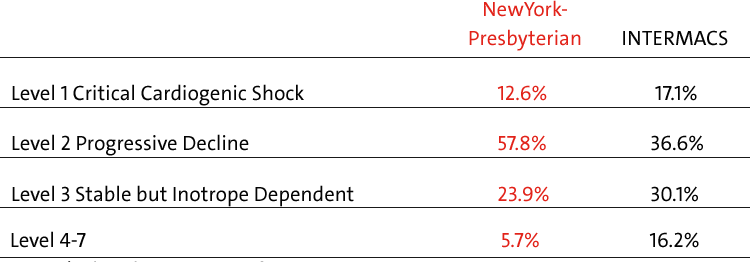
INTERMACS is the United States National Registry for patients receiving durable mechanical
circulatory support device therapy to treat advanced heart failure.
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2016)
Ventricular Assist Device Implants
Volume Distribution, 2016
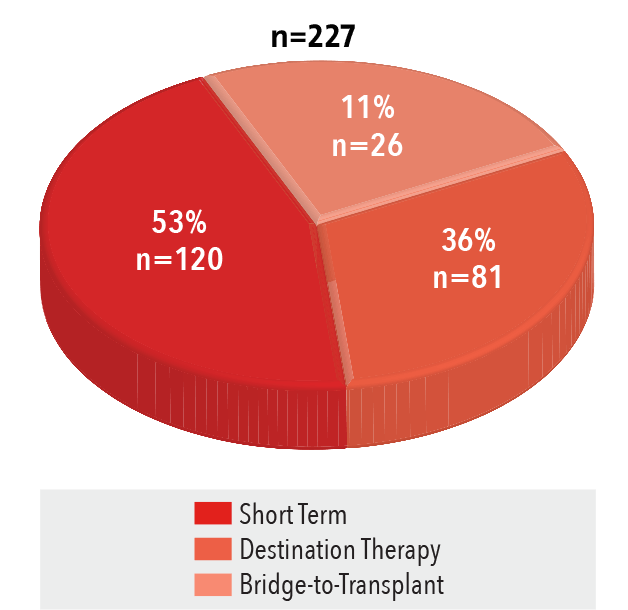
Source: NewYork-Presbyterian
Adverse Events*
June 23, 2006 - December 31, 2016

Table includes overall counts and percentages for each type of adverse event reported at Hospital
site and INTERMACS overall.
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2016)
All Long-Term Implants Post Survival
June 23, 2006 - December 31, 2016
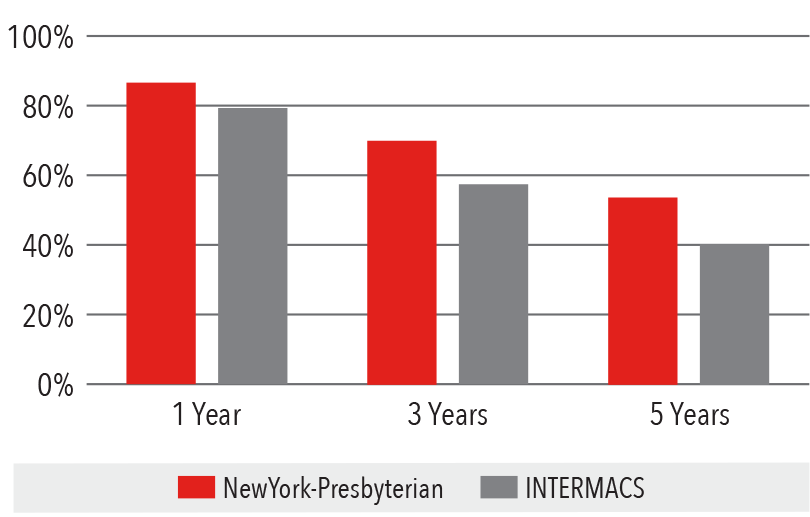
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2016)
Bridge-to-Transplant Survival
June 23, 2006 - December 31, 2016
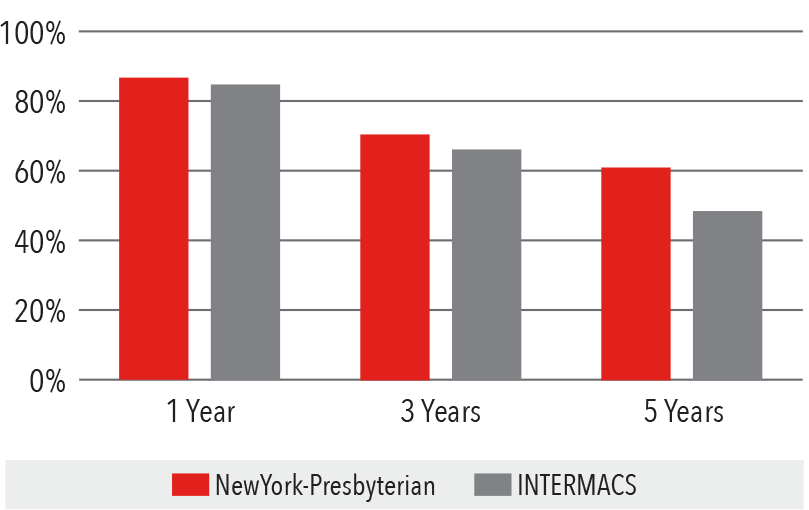
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2016)
Destination Therapy Survival
June 23, 2006 - December 31, 2016
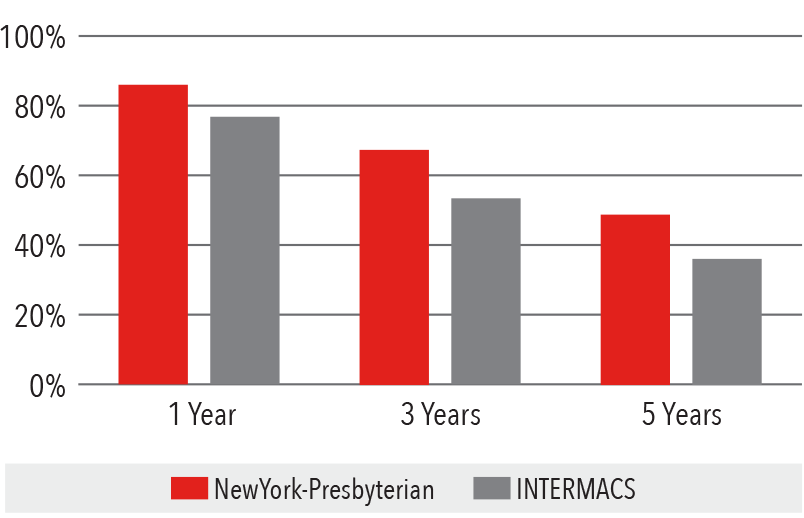
Source: INTERMACS Quality Assurance Quarterly Report (Q4 2016)
Adult Heart Transplant
Patient Survival
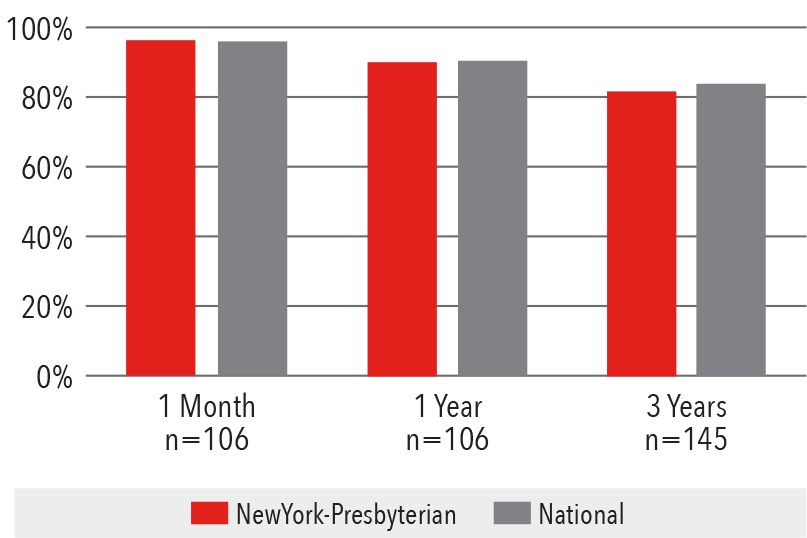
1 month (adjusted) January 1, 2014 - June 30, 2016
1 year (adjusted) January 1, 2014 - June 30, 2016
3 years (adjusted) July 1, 2011 - December 31, 2013
Source: Scientific Registry of Transplant Recipients / srtr.org
Release Date: July 6, 2017
In-Hospital Mortality Rate
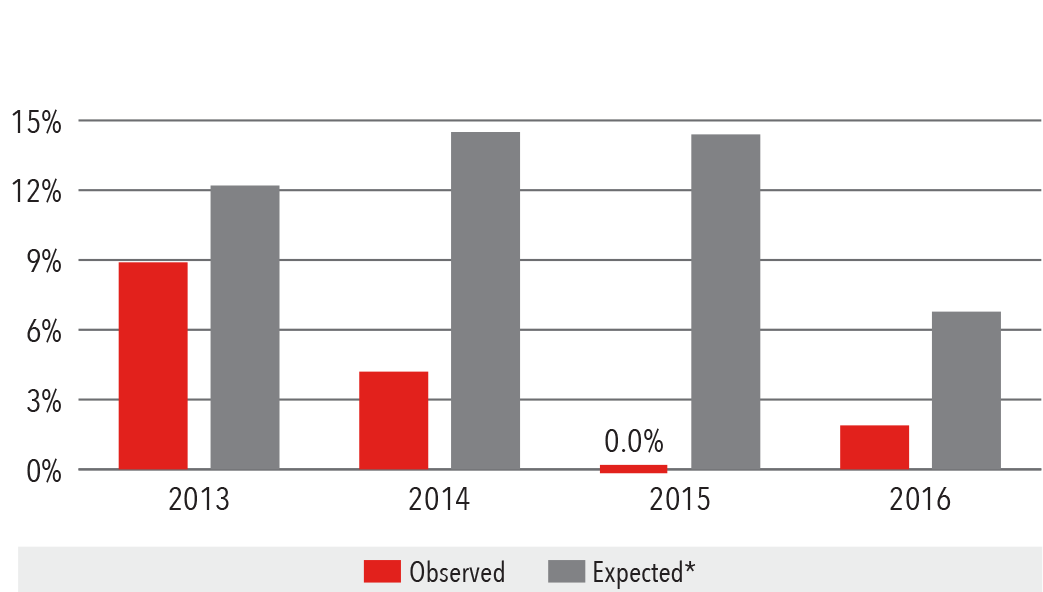
*Expected mortality was determined using Vizient risk-adjustment methodology.
Source: Vizient Clinical Data Base/Resource Manager™
used by permission of Vizient. All rights reserved



