The Big Picture: How Novel Imaging is Transforming Care of Heart Disease
Cardiovascular imaging specialists have always played a key role in the treatment of patients with heart disease. Like their colleagues in cardiology, interventional cardiology, and cardiac surgery, they continually explore and take advantage of the latest technologies and techniques to help understand the mechanisms underlying heart dysfunction and the drivers of patient outcomes.
In the past few years, cardiac imaging has evolved in new and novel ways that are recasting the roles of imagers, who can now be found pursuing pioneering work in the interventional suites, the OR, the research lab. This is particularly evident at NewYork-Presbyterian Hospital, where the cardiac imaging faculty of Columbia University and Weill Cornell Medicine are merging their experience and expertise with those of medical and surgical specialists to advance the treatment of cardiac disease in unprecedented ways.
In the following article, Marco R. Di Tullio, MD; Andrew J. Einstein, MD, PhD; Rebecca T. Hahn, MD; Jiwon Kim, MD; Parmanand Singh, MD; and Jonathan W. Weinsaft, MD, provide an update on cardiac imaging today and a preview of what the future holds.
Clinical Applications: Staying Ahead of the Imaging Curve
First Do No Harm

Dr. Parmanand Singh
Dr. Parmanand Singh, who is dual appointed in Cardiology and Radiology, is Director of the Weill Cornell Nuclear Cardiology Lab and Co-Director of the Cardiac PET Lab. “Working with both cardiologists and radiologists has enabled me to bridge multi-faceted and talented groups to establish productive collaborative efforts with regard to innovative cardiac nuclear imaging protocols and approaches,” says Dr. Singh.
The SPECT (single photon emission computerized tomography) lab offers two state-of-the-art solid-state calcium-zinc-telluride (CZT) cameras that can impressively complete a myocardial profusion scan in three to five minutes in patients with or suspected coronary artery disease. “We transitioned our protocols from the traditional combination of both rest and stress imaging, which had been done for over 20 years, to stress-first and stress-only protocols, whereby we only perform a rest image if necessary,” says Dr. Singh. “The stress images are reviewed promptly by expert faculty and potentially avoids any additional radiation exposure from a second scan, such as rest images, unnecessarily.”
According to Dr. Singh, Weill Cornell’s rest-stress protocols are well below the national average for radiation exposure. “We firmly adhere to the ALARA principle – as low as reasonably achievable,” says Dr. Singh. “With stress-only imaging protocols, the amount of radiation exposure is less than five millisieverts. Our stress imaging protocols coupled with cutting-edge cameras afford the use of very low tracer doses that result in reduced radiation exposure – almost 40 percent of the traditional imaging protocols.” In addition to reducing radiation exposure, shorter imaging times allow for greater patient comfort and satisfaction.

Dr. Andrew J. Einstein
Dr. Andrew J. Einstein is Director of Nuclear Cardiology, Cardiac CT, and Cardiac MRI at NewYork-Presbyterian/Columbia University Irving Medical Center. His research, which is funded by multiple NIH grants, the International Atomic Energy Agency, Columbia’s Zuckerman Institute, and industry, focuses on improving the use of imaging in cardiovascular medicine, with particular interests and current funded projects in patient safety, machine learning, amyloidosis, COVID-19, global health, and device development. He has a particular interest in radiation safety and the development of strategies to minimize risks to patients and practitioners.
“At Columbia, patients undergoing SPECT myocardial perfusion imaging often receive far less radiation than the standard due to the novel protocols and technology we have developed in our laboratory,” says Dr. Einstein. “These include protocols for stress-first and stress-only imaging, which obviate the need for the higher dose resting injection.”
However, while SPECT myocardial perfusion imaging stress-only protocols reduce radiation exposure and cost, they require clinicians to make immediate decisions regarding rest scan cancellation. At Columbia, faculty participated in the development of a machine learning (ML) approach for automatic rest scan cancellation to ultimately use in a clinical setting. In total, 20,414 patients from a solid-state SPECT MPI international multicenter registry with clinical data and follow-up for major adverse cardiac events (MACE) were used to train ML for MACE prediction as a continuous probability. A report from the study, published online in the European Heart Journal Cardiovascular Imaging in June 2020, affirmed that machine learning using clinical and stress imaging data can be used to automatically recommend cancellation of rest SPECT MPI scans, while ensuring higher prognostic safety than current clinical approaches.
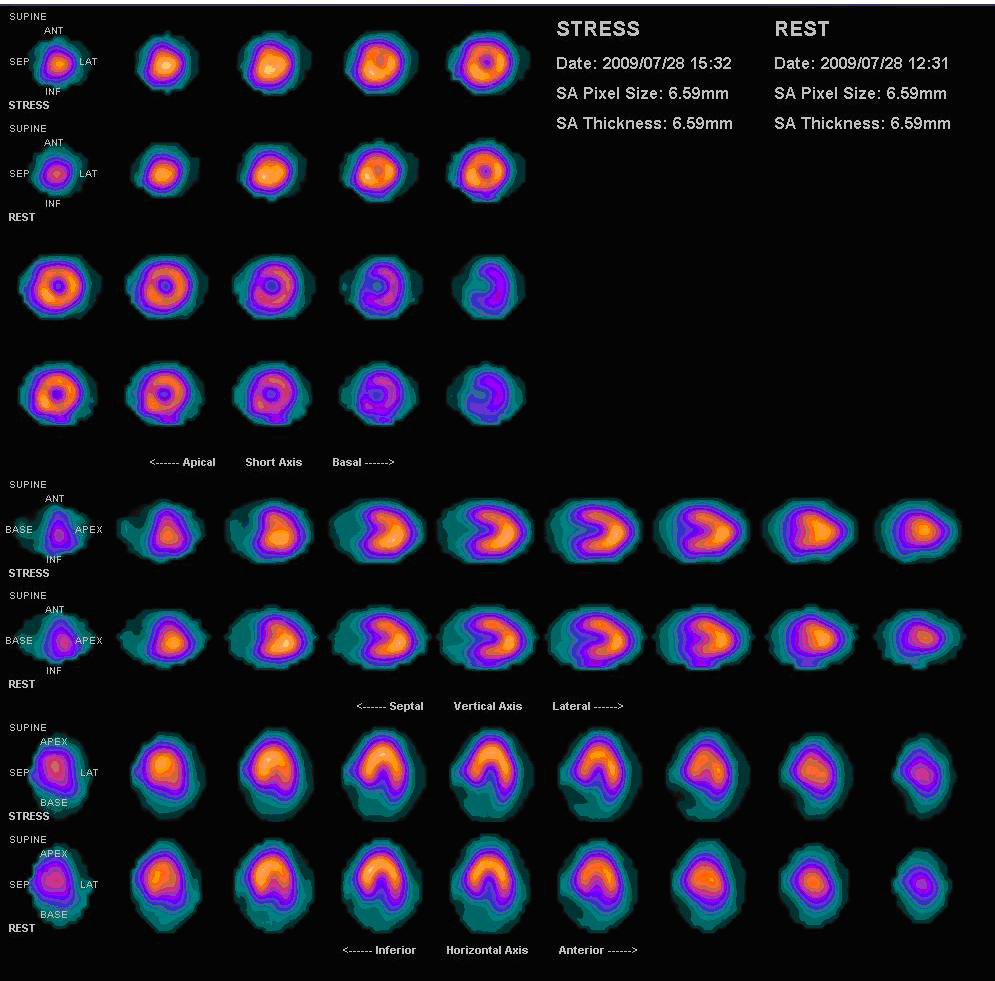
SPECT MPI scan. Click here to view a larger image.
A CT scan with a specialized protocol is critical for patients in preparation for procedures such as TAVR and transcutaneous repair of the mitral or tricuspid valves, but here, too, imagers consider safety first. “We want to be able to minimize the amount of iodinated contrast given to the patient yet provide the salient diagnostic information for physicians to best plan the procedure,” says Dr. Einstein. “With the introduction of TAVR, cardiac imagers at Columbia developed new methods to achieve these goals. We were among the first to perform a single contrast injection for cardiac scanning as well as chest, abdomen, and pelvis scanning, with a minimum amount – as little as 20 or 30 ccs – of contrast by using a volume scanner.”
Innovative Imaging Applications
In the last few years, Columbia faculty introduced the use of pyrophosphate (PYP) imaging for the detection of TTR cardiac amyloidosis. “We work in conjunction with our colleagues in heart failure using SPECT PYP imaging to screen for and diagnose cardiac amyloidosis,” says Dr. Einstein. “We continue to refine and advance our protocols to optimally diagnose and stratify patients.”
Under the leadership of Mathew S. Maurer, MD, Director of Columbia’s Clinical Cardiovascular Research Laboratory for the Elderly, an observational study in collaboration with the National Heart, Lung, and Blood Institute, The Scripps Research Institute, Harlem Hospital Center, and Boston Medical Center, is now underway to screen a cohort of 800 elderly black and Hispanic patients with heart failure for cardiac amyloidosis using nuclear imaging. To refer a patient for the study, contact Dr. Maurer at [email protected].
“Amyloid imaging is key in the diagnostic evaluation of patients with heart failure,” adds Dr. Singh. “When we began offering cardiac PYP scans at Weill Cornell in 2017, we were only performing around 50 scans, however, we are now performing over 250 scans annually.”
In addition, the Cardiac PET Labs at Weill Cornell and Columbia are high volume centers for the evaluation of cardiac sarcoidosis. Both Dr. Singh and Dr. Einstein note that PET cardiac inflammation imaging continues to be pursued for a number of different applications, including the assessment of patients with cardiac infections and, most recently, in developing cardiac-related protocols in the context of COVID-19.
Weill Cornell is also among only a few institutions in the nation to offer hybrid PET-MRI scanning, providing all of the data of a routine MRI in addition to PET information. “For example,” notes Dr. Singh, “combining two imaging techniques into one machine allows for simultaneous and efficient evaluation of cardiac sarcoidosis and other forms of cardiac inflammation.”
At Columbia, faculty have led the development of novel approaches with hybrid imaging to characterize coronary artery calcium that are now widely used across the globe. “Hybrid SPECT- CT and PET-CT studies are a powerful predictor of risk both because of findings in the nuclear images and also because of the attenuation correction scan available in those studies,” says Dr. Einstein. “For SPECT scans, the CT data is used to correct for soft tissue attenuation in the SPECT scans on a slice-by-slice basis thereby improving the accuracy of diagnostic testing, which makes it more likely that we can perform stress-only imaging.”
“Currently, we are very interested in using PET stress testing for a number of reasons,” continues Dr. Einstein. “The test can be performed in our outpatient facility, which, in the era of COVID-19, is important to patients as they don’t need to come into the hospital. It is a highly accurate test and requires a considerably lower radiation dose than SPECT imaging. PET also provides valuable additional information, specifically quantitative data about the amount of myocardial blood under stress and rest conditions. We continue to grow our PET service and have recently developed a novel protocol for PET to enable patients to have their PET scan and stress testing in a single session in a single room.”
In collaboration with bioengineering colleagues at Columbia and elsewhere, Dr. Einstein is involved in a number of efforts to also bring the power of machine learning to improve cardiac diagnosis via multiple imaging modalities.
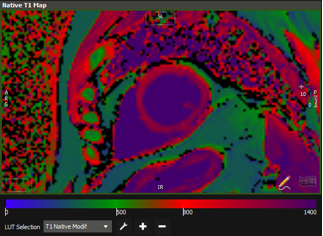
MRI image of the heart of a convalescent COVID-19 patient
Most recently, Dr. Einstein, Dr. Di Tullio, and their colleagues J. Thomas “Tommy” Vaughan, Jr., PhD, Director of Magnetic Resonance Research, Columbia Engineering, and Andrew F. Laine, DSc, Director of the Heffner Biomedical Imaging Lab at Columbia, have initiated a study to assess the impact of COVID-19 on myocardial structure and function using Clariscan-enhanced cardiovascular magnetic resonance (CMR).
The COvid-19 LongitUdinal Multiethnic BioImaging Assessment of CARDiovascular Sequelae (COLUMBIA CARDS) Registry Pilot Study is providing a multi-sequence CMR and echocardiography evaluation of a multiethnic spectrum of convalescent COVID-19 patients in comparison to controls. Their goal is to generate pilot data for a larger grant proposal to study the relationships between myocardial characteristics by CMR and echocardiography and health outcomes, and how these are modulated through patient characteristics, clinical characteristics of acute COVID-19 illness, and serologic biomarkers. “This myocardial characterization will not just provide diagnosis, but also serve as a potentially powerful tool for risk stratification, therapeutic decision making, and monitoring response to therapies in COVID-19 survivors,” says Dr. Einstein. To refer a patient for this study, contact Efrain “Frankie” DeJesus at [email protected].
Cardiac Imaging in the Structural Disease Space

Dr. Rebecca T. Hahn
Since joining the Columbia faculty in 2007, Dr. Rebecca T. Hahn, Director of Interventional Echocardiography at NewYork-Presbyterian/Columbia, has been paving the way for new roles for cardiac imagers. At the time, structural heart disease was a fledgling field and Dr. Hahn primarily worked in the echocardiography laboratory. “Structural heart disease was starting to develop as a new subspecialty within cardiology,” notes Dr. Hahn. “The PARTNER trial had started as well as a trial for the mitral valve. With both these trials underway at Columbia, it became clear that an imager was going to be needed to work intra-procedurally. Dr. Leon insightfully took me out of the echo lab and basically hired me for the interventional lab. It was the first time that an imager had been incorporated into the interventional team.”
Under the leadership of Martin B. Leon, MD, Director of the Center for Interventional Vascular Therapy at Columbia, this new interventional team model became the foundation of consensus manuscripts advocating for a multimodality, multidisciplinary team of physicians working toward a common goal. “It’s not that this model had not been used before in other specialties, but it had not really been used in cardiology,” says Dr. Hahn. “It was the brilliant brainchild of Dr. Leon and completely changed the field. Cardiac programs began to incorporate the imager into structural heart disease interventions, which, in turn, opened the door to the potential of new, more complex procedures and application of new devices now made possible with imaging guidance.”
“The development and the advance of structural heart disease, I think, in part, is based on the availability of advanced imaging tools for detecting structural and valve pathology, quantifying severity, and guiding therapy,” continues Dr. Hahn. “The interventional echocardiographer supports pre-procedural patient selection, participates in decision-making intra-procedurally, particularly with positioning the device, and provides continuous procedural monitoring. For all these reasons the imager has become such an integral member of the heart team for structural heart disease.”
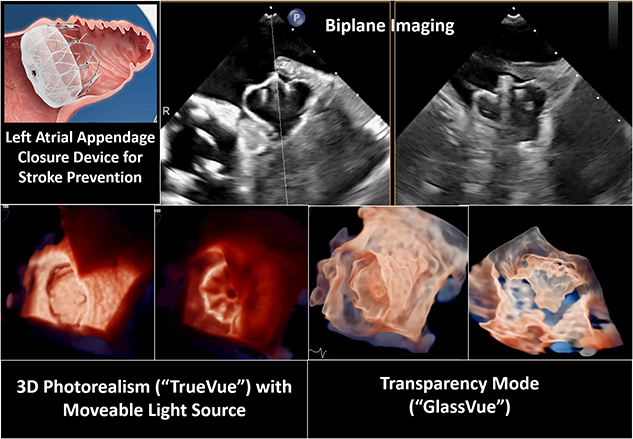
Advanced three-dimensional echocardiographic imaging tools
With ultrasound evolving from a diagnostic procedure to a technology used to guide the deployment of devices in transcatheter procedures, Columbia cardiac imaging specialists also began working closely with ultrasound companies, testing new ultrasound image and workflow enhancements specifically for the structural space, as well as with device companies, where the imager’s comprehensive understanding of anatomy helps the developers understand the limitations of the structural anatomy of interest.
The cardiac faculty at Columbia were among the first investigators to become interested in the tricuspid valve. They have worked extensively in developing safe, minimally invasive interventions to successfully treat tricuspid valve disease and are leading the major clinical trials of transcatheter devices and procedures. This includes the early feasibility SCOUT trial, which evaluated an investigational percutaneous tricuspid valve system, TriAlign, for symptomatic chronic functional tricuspid regurgitation. The study demonstrated that the transcatheter plication device achieved 93 percent procedural success with no procedural mortality or stroke; successful delivery and retrieval of the device delivery system with proper device placement in all patients; and improvements in outcomes related to tricuspid regurgitation severity and quality-of-life measurements.
“With the SCOUT trial, we investigated new ways of quantifying and grading tricuspid regurgitation severity. In our initial manuscript, we were able to show a 1 or 2 grade reduction in tricuspid regurgitation that was significantly associated with an improvement in functional status and quality of life,” says Dr. Hahn, who was first author on the article published in the April 2017 issue of the Journal of the American College of Cardiology. “The SCOUT trial was the first of its kind. Since introducing the extended grading scheme for this disease, numerous papers have documented increasing mortality associated with higher grades of tricuspid regurgitation beyond severe, what we called ‘massive’ and ‘torrential.’ Therefore, being able to reduce tricuspid regurgitation by 2 grades to severe is actually impactful for those patients.”
At Columbia, newer clinical trials for nonsurgical treatment of mitral and tricuspid valve disease are well underway. Dr. Hahn emphasizes that the success of these newer interventional procedures relies on the ability to perform a high-quality computed tomography angiography examination, which she and her colleagues discuss in detail in the March 2019 issue of JACC: Cardiovascular Imaging. The authors note, “CTA offers the potential for detailed anatomic assessment in this patient population, but its optimal implementation for patients with mitral and tricuspid disease requires patient-centered protocol specification, an understanding of complex anatomy and pathophysiology, and the particulars of CT scanner capabilities.”
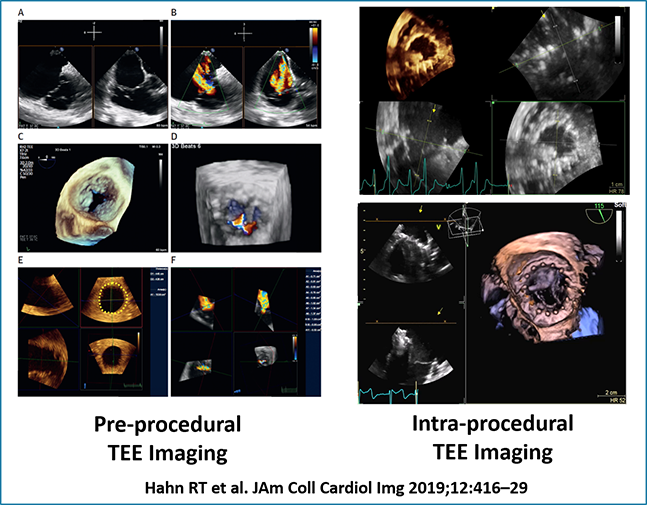
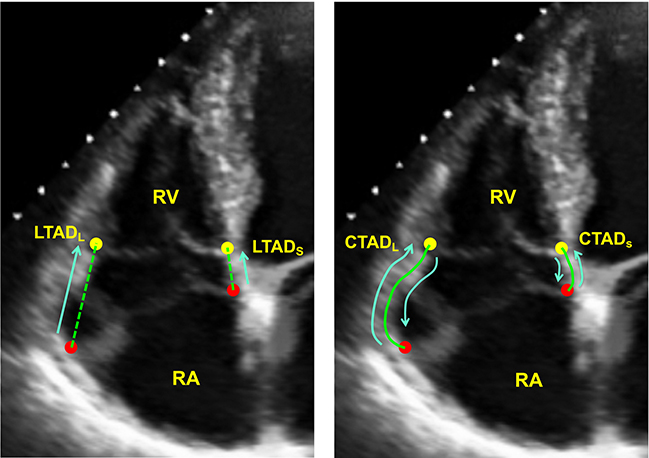
Intraprocedural imaging for imaging for a novel transcatheter tricuspid valve replacement device (JACC: Cardiovascular Imaging, March 2019)
In a recent study, Dr. Hahn and her colleagues evaluated three-dimensional transesophageal echocardiographic (TEE) imaging, which is frequently used as an initial screening tool in the evaluation of patients who are candidates for transcatheter tricuspid valve replacement. The researchers found that 3D TEE measurements of tricuspid annular dimensions display strong correlations with CT measurements in patients undergoing screening and therefore should be proposed as a reasonable alternative to CT imaging in this vulnerable population.
MRI Tissue Characterization: Understanding Mitral Regurgitation

Dr. Jonathan W. Weinsaft
Dr. Jonathan Weinsaft and his colleagues are studying the interactions between myocardial fibrosis and mitral valve disease to understand mechanisms by which both focal and diffuse fibrosis can increase loading pressures on the mitral valve, ultimately leading to mitral regurgitation and mitral valve failure. “We are looking at that in different contexts, the first being patients with ischemic or functional mitral regurgitation,” says Dr. Weinsaft, noting that functional mitral regurgitation occurs in nearly half a million people in the United States every year, and each year doubles the risk for heart failure leading to death. “We know that reasons for functional mitral regurgitation are not the valve itself, but abnormal changes in shape and structure of the left ventricular myocardium.”
Using MRI tissue characterization, the physicians examine fibrosis in the heart underlying the mitral valves, both the papillary muscles and supporting structures. They seek to learn whether fibrosis and related ischemia in new blockages in that valve are responsible for mitral regurgitation, the key drivers for change in mitral regurgitation after coronary revascularization. “We’re also looking at the way those tissue characteristics impact response to incremental therapy, specifically the angioplasty approach or MitraClip™, a growing percutaneous approach to treat degenerative and functional mitral regurgitation,” says Dr. Weinsaft.
In addition, Dr. Weinsaft and his colleagues are investigating whether loading stresses on the mitral valve, as well as on subvalvular fibrosis, are key determinants to tailor and to guide therapy. “Our ultimate goal is to better select those patients in whom we’re going to effect a durable reduction of mitral regurgitation, and ultimately an improved outcome with a given therapy, whether it be revascularization, MitraClip, or angioplasty.”
Focusing on the Forgotten Ventricle

Dr. Jiwon Kim
“Most cardiologists and physicians have generally focused on the left heart and left ventricular function, but over the years, we’ve shown that the right heart is just as important a structure, if not more important, than the left heart in some cardiac conditions,” says Dr. Jiwon Kim, whose clinical and research interest is focused on valvular heart disease and cardiomyopathies using cardiac MRI, cardiac CT, and 3D echocardiography. “If the right heart is failing, the risk of mortality goes up substantially even if the left heart function is normal. I have felt that developing new ways to assess the right heart with a goal of improving right heart function in patients with heart disease is critical.”
To improve visualization of the right heart Dr. Kim and her colleagues are applying methods used to view the left ventricle. “My work primarily entails using 3D echocardiography and strain echocardiography with speckle tracking, which outperforms all of the other existing indices that we have to assess the right ventricle,” says Dr. Kim, who has tested the magnitude of agreement between echocardiography and cardiac magnetic resonance-derived left atrial strain and their relative diagnostic performance in discriminating diastolic dysfunction and predicting atrial fibrillation. The results were published in the August 2020 issue of JACC: Cardiovascular Imaging.
“We have validated the strain echo tracking technique in being able to predict prognosis in patients. Strain is the index of choice to predict heart failure and right ventricular failure perioperatively,” says Dr. Kim.
The Weill Cornell physicians have also developed machine learning techniques to segment the right ventricle in which they can process thousands of echoes quickly. They conducted a multimodality validation study of a novel machine learning model for right ventricular quantification on echocardiography, the results of which were published in the May 2020 issue of Echocardiography. “This is one of the first studies using machine learning applications for the right ventricle,” says Dr. Kim. “We can analyze many images in seconds, avoiding a lot of the pitfalls associated with conventional techniques for right ventricular evaluation. Now that we have the ability to analyze echoes rapidly in a systematic fashion, we’re looking to apply this technique to analyze large data sets. That’s been very exciting.”
Weill Cornell cardiac imaging specialists provide an initial validation of a deep learning system for right ventricle assessment using automated tracking of the tricuspid annulus. This figure demonstrates representative lateral and septal annular displacement performed both manually and by machine learning algorithm. (Echocardiography, May 2020)
There is existing data regarding left ventricular strain and cancer patients. Now the Weill Cornell team is also studying right ventricular strain in patients with cancer. “We’ve shown that it actually has incremental prognostic value in terms of its ability to predict death,” says Dr. Kim. Results of their study were published in December 2019 issues of the Journal of the American Society of Echocardiography.
“We are also seeking ways to improve right heart function in patients whose right ventricles are dysfunctional,” adds Dr. Kim, whose K23 NIH grant is testing the concept of myocardial tissue characterization guided coronary revascularization as a means to improve right heart function. “We’ve shown that patients with coronary artery disease as well as the underlying ischemia have high rates of right ventricular dysfunction. We want to look at the impact of relieving the ischemia and its impact on the right heart. We have preliminary data that revascularization not only helps the left heart, but also improves function of the right heart. That is particularly relevant because our current therapies for the right heart are very limited. Adding this new application has the potential to try to improve right heart function for many patients.”
Subclinical Cardiovascular Abnormalities as Predictors of Stroke

Dr. Marco R. Di Tullio
As Director of Echocardiography Research and Associate Director of Cardiovascular Ultrasound Laboratories at NewYork-Presbyterian/Columbia, Dr. Marco R. Di Tullio straddles two domains. At the clinical level, he applies his imaging expertise to relating findings to existing disease. At the subclinical level, he is interested in detecting subtle abnormalities that can predispose an individual to developing the disease in order to identify an earlier subclinical stage when an intervention can be most effective.
For many years, Dr. Di Tullio, funded by NIH grants, studied new possible cardiac abnormalities that predispose individuals to stroke. “Essentially, I was looking at stroke as possibly originating from a cardiac source – such as patent foramen ovale, or PFO,” says Dr. Di Tullio, who, in 1992, authored the first article published in the United States on the risk of stroke associated with PFO. “Even to these days, the most crucial question that remains is how do we differentiate those individuals with PFO who go on to have a stroke from the majority of people with PFO who will never have a stroke? Some progress has been made in this field, but more remains to be clarified.”
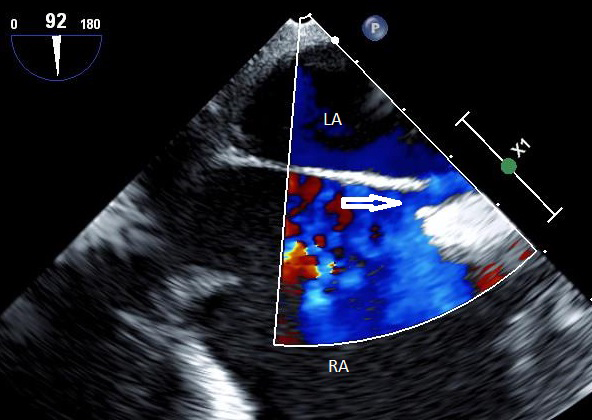
Visualization of PFO by transesophageal echocardiography. The PFO opening is shown (arrow), with blood shunting between the atria (in blue). LA = Left atrium RA = Right atrium (Courtesy of Dr. Marco Di Tullio)
In recent years, Dr. Di Tullio transitioned the focus of his investigations from the clinical to the subclinical domain, using echocardiography as a tool to answer epidemiological questions. “Over the years, I received several NIH grants to investigate the relationship between subclinical abnormalities of the heart, blood pressure, vascular distensibility, and other factors that can be evaluated with ultrasound to determine how they relate to risk of subclinical brain disease, which in turn predisposes to stroke. The current focus of my research is on the association between subtle abnormalities of the heart and the presence of signs of vascular disease in the brain,” notes Dr. Di Tullio. “Does subclinical cardiovascular disease predispose individuals to an increased risk of stroke? If we find a relationship between subclinical abnormality of the heart and subclinical abnormality of the brain, we may have found another possible risk factor for stroke that may be intervened on at an earlier time.”
Subclinical silent cerebrovascular disease, defined as silent brain infarctions and white matter hyperintensity volume, is frequently observed on MRI in older adults who do not present with obvious neurological symptoms. Approximately 1,000 predominantly older participants in the Cardiovascular Abnormalities and Brain Lesions (CABL) study underwent two-dimensional echocardiography, arterial wave reflection assessment, analysis for determination of 24-hour BP and derived central BP, and brain MRI. The original CABL study, published in Circulation in 2013, had showed that a reduction in left ventricular global longitudinal strain, an indicator of subclinical ventricular dysfunction, was associated with subclinical brain disease detected by brain MRI in subjects without history of stroke, even in the presence of still normal global ventricular function. Also, larger left atrial volumes and reduced left atrial reservoir function were independently associated with subclinical cerebrovascular disease (JACC: Cardiovascular Imaging, 2013), as were abnormal left ventricular mass and geometry (American Heart Journal, 2017) and nocturnal blood pressure (European Heart Journal Cardiovascular Imaging, 2019).
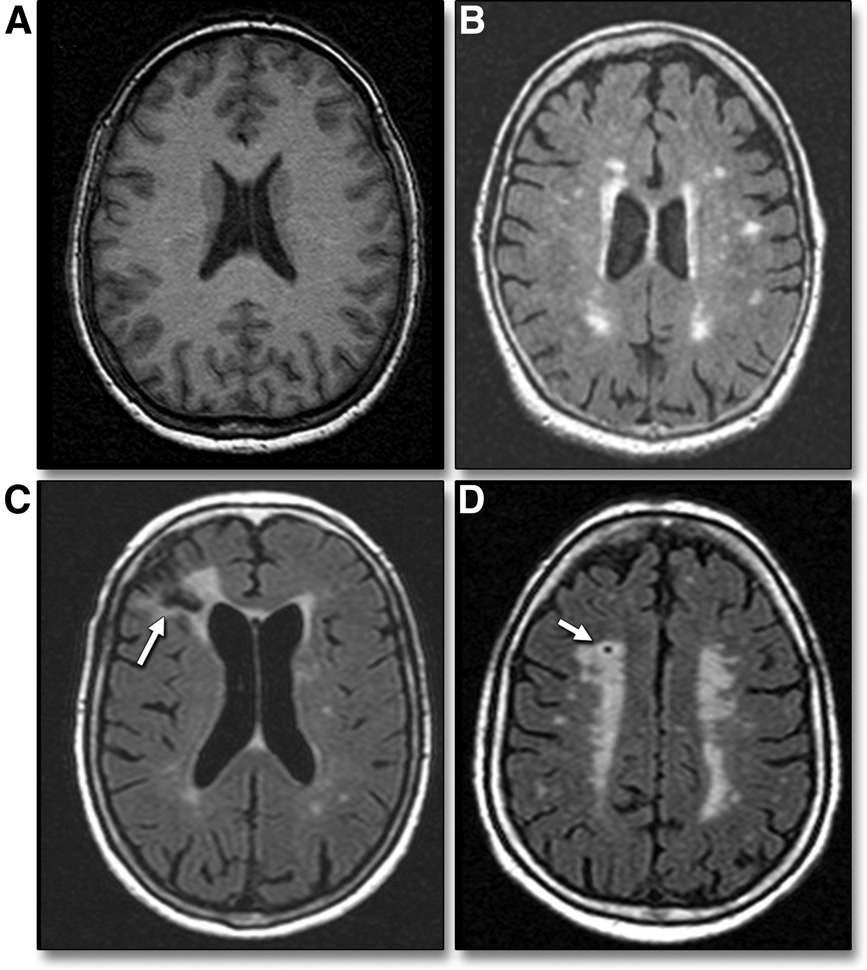
Representative axial MRI slices from 2013 CABL study participants showing (A) normal brain and subclinical ischemic changes, including (B) white matter hyperintensities, (C) cortical infarction (arrow), and (D) lacunar infarction (arrow). (JACC: Cardiovascular Imaging, March 2013)
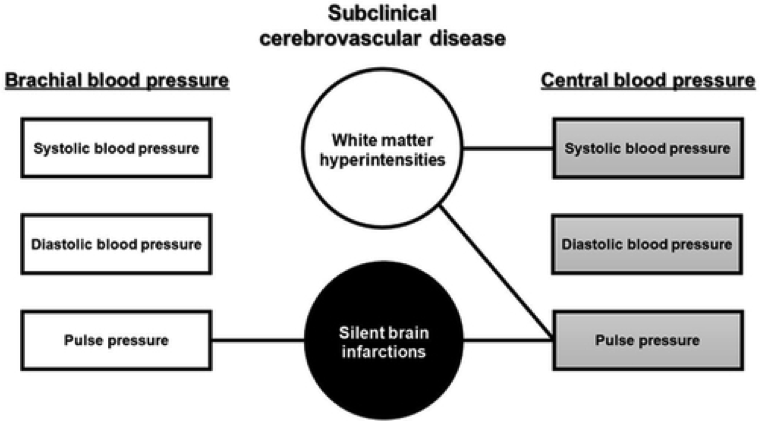
In a recent study published in Hypertension, Dr. Di Tullio and his colleagues examined the association between central blood pressure, derived from peripheral vascular tonometry, and silent cerebrovascular disease, which had not been clearly established previously. The study showed that while both brachial and central pulse pressures were independently associated with silent brain infarctions; only higher central systolic BP and central pulse pressure, but not brachial BP, were significantly associated with white matter hyperintensity volume, another indicator of stroke risk. Therefore, central blood pressure determination, used in lieu of the commonly measured brachial blood pressure, might refine the prediction of subclinical brain disease, with possible preventative implications.
In the most recent phase of his NIH grant, Dr. Di Tullio is supplementing echocardiographic information with assessment of cardiac rhythm through 14-day continuous monitoring with a patch-based recording device. “It is known that atrial fibrillation is a strong risk factor for stroke; however, it is not known how many people have silent unsuspected episodes of atrial fibrillation and, therefore, may be at increased risk of stroke without knowing it,” explains Dr. Di Tullio. “In the Subclinical Atrial Fibrillation and Risk of Ischemic Stroke (SAFARIS) study, I am looking at the frequency of atrial fibrillation and other cardiac arrhythmias in the general elderly population to identify whether subclinical cardiac abnormalities, such as reduced left ventricular or left atrial strain, may predispose to atrial fibrillation and other arrhythmias, which in turn predispose to stroke.
Novel Imaging for Assessing Prosthetic Graft Replacement
In collaboration with the Weill Cornell Department of Cardiothoracic Surgery, Dr. Jonathan Weinsaft and his colleagues are conducting a prospective study funded by The Marfan Foundation using new imaging approaches to evaluate the impact of ascending aortic grafting on wall shear stress and compliance of the native descending thoracic aorta. “Prosthetic grafting of ascending aortic aneurysms offers lifesaving benefits,” says Dr. Weinsaft. “We also know that there are some patients with genetically mediated aneurysms in whom the vessel wall properties are abnormal, and despite immediate lifesaving benefits of aortic grafting, there is a residual long-term repercussion or recurring aneurysm and rejection of the prosthetic graft.”
The researchers are focused on interactions between the prosthetic graft and native aorta, specifically that the ascending graft serves as a conduit to propagate high-energy flow to the native descending aorta. “We are using techniques such as new MRI pulse sequences and four-dimensional flow imaging to visualize complex flow in the aorta as well as flow to the graft,” says Dr. Weinsaft. “This is high resolution, late gadolinium enhancement imaging, which enables us to look at fibrotic scarring in areas of the heart that we never could see before.”
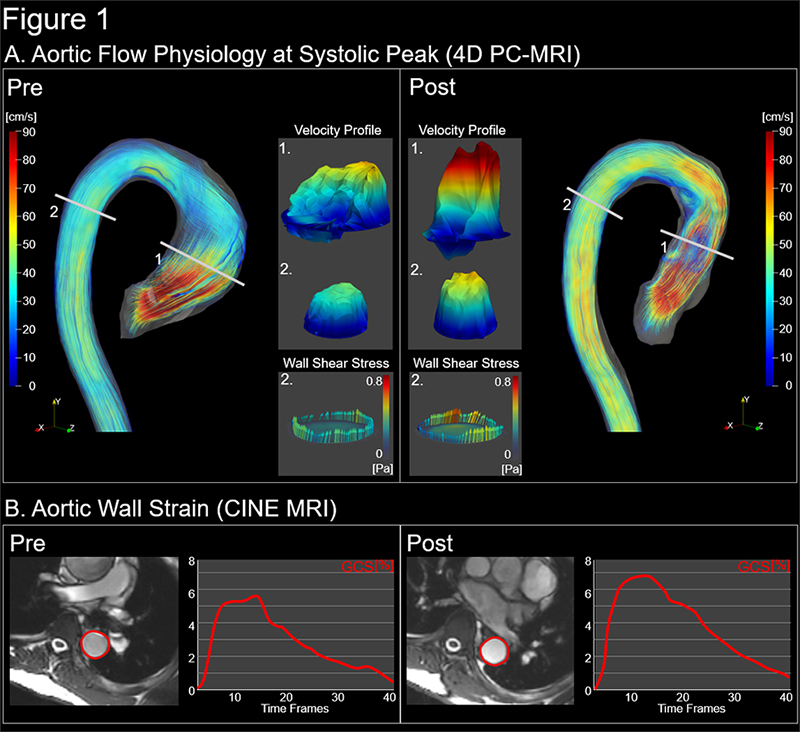
(A) Aortic Flow physiology at systolic peak - 4D PC-MRI (B) Aortic wall strain - CINE MRI
(Courtesy of Dr. Jonathan Weinsaft). Click here to view a larger image.
In other studies, the team is using computational modeling to take primary imaging data and perform simulation to test whether modifying the features of an aortic graft or the features of a mitral valve replacement could alter outcome and ultimately improve therapies for these patients. “The goal would be to extend those simulations to actual clinical care, but this is a critical first step,” says Dr. Weinsaft.
Reference Articles
Kim J, Yum B, Palumbo MC, Sultana R, Wright N, Das M, You C, Moskowitz CS, Levine RA, Devereux RB, Weinsaft JW. Left atrial strain impairment precedes geometric remodeling as a marker of post-myocardial infarction diastolic dysfunction. JACC: Cardiovascular Imaging. 2020 Aug 16:S1936-878X(20)30617-3. [Epub ahead of print]
Rong LQ, Rahouma M, Lopes A, Devereux RB, Kim J, Pryor KO, Girardi LN, Weinsaft JW, Gaudino MFL. Differential myocardial strain in the early postoperative period in patients receiving arterial vs venous bypass grafts: A hypothesis-generating study. Journal of Cardiac Surgery. 2020 Aug;35(8):1824-31.
Coisne A, Pontana F, Aghezzaf S, Mouton S, Ridon H, Richardson M, Polge AS, Longère B, Silvestri V, Pagniez J, Bical A, Rousse N, Overtchouk P, Granada JF, Hahn RT, Modine T, Montaigne D. Utility of three-dimensional transesophageal echocardiography for mitral annular sizing in transcatheter mitral valve replacement procedures: A cardiac computed tomographic comparative study. Journal of the American Society of Echocardiography. 2020 Jul 25:S0894-7317(20)30309-6.
Plodkowski AJ, Chan A, Gupta D, Lakhman Y, Kukar N, Kim J, Perez-Johnston R, Ginsberg MS, Steingart RM, Weinsaft JW. Diagnostic utility and clinical implication of late gadolinium enhancement cardiac magnetic resonance for detection of catheter associated right atrial thrombus. Clinical Imaging. 2020 Jun;62:17-22.
Hu LH, Miller RJH, Sharir T, Commandeur F, Rios R, Einstein AJ, Fish MB, Ruddy TD, Kaufmann PA, Sinusas AJ, Miller EJ, Bateman TM, Dorbala S, Di Carli M, Liang JX, Eisenberg E, Dey D, Berman DS, Slomka PJ. Prognostically safe stress-only single-photon emission computed tomography myocardial perfusion imaging guided by machine learning: report from REFINE SPECT. European Heart Journal Cardiovascular Imaging. 2020 Jun 12:jeaa134. [Online ahead of print]
Coisne A, Pontana F, Modine T, Sudre A, Lancellotti P, Hahn RT, Khalique OK, Granada JF, Montaigne D, Donal E. Transcatheter mitral valve replacement guided by echocardiographic-CT scan fusion: Early human clinical experience. JACC: Cardiovascular Interventions. 2020 Jun 8;13(11):1376-78.
Beecy AN, Bratt A, Yum B, Sultana R, Das M, Sherifi I, Devereux RB, Weinsaft JW, Kim J. Development of novel machine learning model for right ventricular quantification on echocardiography: A multimodality validation study. Echocardiography. 2020 May;37(5):688-97.
Zhang Y, Wang VY, Morgan AE, Kim J, Ge L, Guccione JM, Weinsaft JW, Ratcliffe MB. A novel MRI-based finite element modeling method for calculation of myocardial ischemia effect in patients with functional mitral regurgitation. Frontiers in Physiology. 2020 Mar 13;11:158.
Pulerwitz TC, Khalique OK, Leb J, Hahn RT, Nazif TM, Leon MB, George I, Vahl TP, D'Souza B, Bapat VN, Dumeer S, Kodali SK, Einstein AJ. Optimizing cardiac CT protocols for comprehensive acquisition prior to percutaneous MV and TV repair/replacement. JACC: Cardiovascular Imaging. 2020 Mar;13(3):836-50.
Matsumoto K, Jin Z, Homma S, Elkind MSV, Rundek T, Mannina C, Lee TC, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Association between central blood pressure and subclinical cerebrovascular disease in older adults. Hypertension. 2020 Feb;75(2):580-87.
Kim J, Krichevsky S, Xie L, Palumbo MC, Rodriguez-Diego S, Yum B, Brouwer L, Silver RT, Schafer AI, Ritchie EK, Yabut MM, Sosner C, Horn EM, Devereux RB, Scandura JM, Weinsaft JW. Incremental utility of right ventricular dysfunction in patients with myeloproliferative neoplasm-associated pulmonary hypertension. Journal of the American Society of Echocardiography. 2019 Dec;32(12):1574-158.
Hahn RT, Nabauer M, Zuber M, Nazif TM, Hausleiter J, Taramasso M, Pozzoli A, George I, Kodali S, Bapat V, Maisano F. Intraprocedural imaging of transcatheter tricuspid valve interventions. JACC: Cardiovascular Imaging. 2019 Mar;12(3):532-53.
Nakanishi K, Jin Z, Homma S, Elkind MS, Rundek T, Tugcu A, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Left ventricular mass-geometry and silent cerebrovascular disease: The Cardiovascular Abnormalities and Brain Lesions (CABL) study. American Heart Journal. 2017 Mar;185:85-92.



