Aneuploidy is a major reason why embryos derived in vivo and in vitro fail to implant or result in a healthy pregnancy. According to a new study from researchers at NewYork-Presbyterian/Weill Cornell Medicine, an artificial intelligence (AI) algorithm can determine non-invasively, with about 70 percent accuracy, if an in vitro fertilized embryo has a normal or abnormal number of chromosomes.
Preimplantation genetic testing, one of the current methods for detecting aneuploidy, involves biopsy-like sampling and genetic testing of cells from a developing embryo – an approach that adds cost to the in vitro fertilization (IVF) process and is invasive to the embryo. STORK-A, the new AI algorithm developed and tested by NewYork-Presbyterian/Weill Cornell Medicine faculty, , can help predict aneuploidy by noninvasively analyzing microscope images of the embryo and incorporating information about maternal age and the IVF clinic’s scoring of the embryo’s appearance.
The research team included Nikica Zaninovic, MS, PhD, Associate Professor of Embryology in Clinical Obstetrics and Gynecology and Director of the Embryology Laboratory at the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine at NewYork-Presbyterian/Weill Cornell Medicine; Zev Rosenwaks, MD, Director and Physician-in-Chief of the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine; Olivier Elemento, PhD, Director of the Englander Institute for Precision Medicine and a Professor of Physiology and Biophysics and of Computational Genomics in Computational Biomedicine at Weill Cornell Medicine; Iman Hajirasouliha, PhD, Associate Professor of Computational Genomics and of Physiology and Biophysics and a member of the Englander Institute; and Josue Barnes, a doctoral student in the Weill Cornell Graduate School of Medical Sciences who studies in the Hajirasouliha Laboratory.
A Computational Approach to Embryo Assessment
Currently, fertility specialists currently use microscopy to assess embryos for morphological abnormalities that correlate with poor viability. To obtain information about the chromosomes, clinicians may also use a biopsy method – preimplantation genetic testing for aneuploidy (PGT-A) – predominantly in women over the age of 37.
At NewYork-Presbyterian/Weill Cornell Medicine, investigators from the Center for Reproductive Medicine teamed up with colleagues in the Englander Institute to develop a computational approach to embryo assessment that capitalized on the embryology laboratory’s pioneering use of time lapse photography. Their work began with a 2019 study in which the researchers developed an AI algorithm, STORK, that could assess embryo quality mimicking IVF specialists. For their current study, the Weill Cornell Medicine researchers developed STORK-A as a potential alternative for PGT-A or as a more selective way of deciding which embryos should have PGT-A testing.
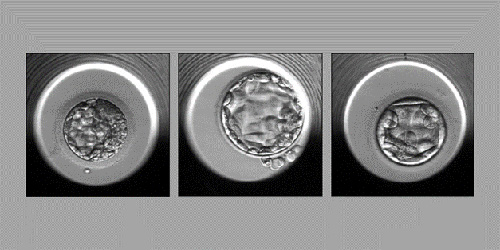
Examples of embryos evaluated by the STORK-A algorithm (from left): an embryo predicted to have an abnormal chromosome count or a single chromosomal abnormality; an embryo predicted to have a normal chromosome count; and an embryo predicted to have more than one chromosomal abnormality. (Credit: The Lancet. Digital Health 2023)
Deep-learning approaches have evolved over the past decade as a powerful tool for tasks such as image classification and are valuable for the analysis of embryonic imaging data. The new STORK-A algorithm uses microscope images of embryos taken at five days post fertilization when scoring of embryo quality, maternal age, and other information is generally gathered as part of the IVF process. Because it uses AI, the algorithm automatically correlates certain features of the data – often too subtle for the human eye – with the chance of aneuploidy.
“We believe that ultimately by using this technology we can reduce the number of embryos to be biopsied, reduce the costs, and provide a very good tool for consultation with the patient when they need to make a decision whether to do PGT-A or not.” – Dr. Nikica Zaninovic
Testing the Significance of STORK-A
In their retrospective study of STORK-A, the results of which were published in the January 2023 issue of The Lancet. Digital Health, the team trained STORK-A on a dataset of 10,378 embryos – all of which had PGT-A results – from 1,385 patients. STORK-A predicted aneuploid versus normal chromosome euploid embryos at nearly 70 percent (69.3 percent). A second classification task trained to predict complex aneuploidy (two or more abnormal chromosomes) versus euploidy and single aneuploidy produced an accuracy of 74 percent, while a third task to predict complex aneuploidy versus euploidy showed a 77.6 percent accuracy. The team later tested the algorithm on independent datasets, including one from an IVF clinic in Spain, and found comparable accuracy results, demonstrating the generalizability of STORK-A.
The STORK-A study provides a proof of concept for an approach that is currently experimental. Standardizing the use of STORK-A in clinics would require clinical trials comparing it to PGT-A and Food and Drug Administration approval – all years in the future. But the new algorithm represents progress on the way to making IVF embryo selection less risky, less subjective, less costly, and more accurate.
“We believe that ultimately by using this technology we can reduce the number of embryos to be biopsied, reduce the costs, and provide a very good tool for consultation with the patient when they need to make a decision whether to do PGT-A or not,” says Dr. Zaninovic.
“This is another great example of how AI can potentially transform medicine,” adds Dr. Elemento. “The algorithm turns tens of thousands of embryo images into AI models that may ultimately be used to help improve IVF efficacy and further democratize access by reducing costs.”
“This study suggests a future role for STORK-A in the fertility clinic,” the authors noted. “However, STORK-A in its current state is not intended to be a derivative of a prenatal test or replace PGT-A, as further development and randomized clinical studies would be required beforehand. Rather, STORK-A is intended to be an assistive decision-making tool that provides a standardized, noninvasive, and cost-efficient means of selecting and prioritizing high quality embryos for PGT-A biopsy or transfer to patients as opposed to using traditional methods such as morphological assessment, which are biased and subjective.”
The Weill Cornell Medicine team now plans to build on this success with algorithms trained on videos of embryo development alone (without human evaluation). “This technology is being optimized with the hope that at some point its accuracy will be close to genetic testing, which is the gold standard and is more than 90 percent accurate,” notes Dr. Rosenwaks.



