
Dr. Andrew Lenis
Research on understanding the biology of bladder cancer, the fifth most prevalent cancer in the United States, remains limited. Andrew T. Lenis, MD/MS, a surgeon-scientist and urologic oncologist in the Department of Urology at NewYork-Presbyterian/
“Translational research is so important in our fight against cancer,” says Dr. Lenis, who specializes in urothelial cancer. “The problems that we are trying to tackle require not only team science, but also translational investigators who understand the clinical problems, know the clinical questions to ask, bring those questions to the lab, and then work with the basic scientists to address these questions.”
Dr. Lenis, who joined Columbia in 2022 after completing a fellowship in urologic oncology at Memorial Sloan Kettering Cancer Center, divides his time evenly between his practice and his research in the Shen Lab. “Clinically and from a lab standpoint my focus is on bladder cancer,” says Dr. Lenis. “More than 80,000 people will be diagnosed this year in the United States with bladder cancer and about 75 percent have a non-invasive form of the disease. While those tumors tend to recur, they can be treated with endoscopic resection and intravesical organ sparing treatment. But about 25 percent of these tumors are invasive. These potentially lethal tumors require more radical therapy, including chemotherapy, bladder removal, and urinary diversion, which greatly impacts quality of life.”
Dr. Lenis’s research focuses on the transition from non-invasive tumors to invasive tumors. “That process is very important because if we understand that pathway, then we can target therapies to try and limit that process of progression,” he says.
In a paper published in the April 5, 2018, issue of Cell, Dr. Shen and his lab team demonstrated that 3D bladder cancer organoids would essentially recapitulate the genomic alterations found in the parental tumor, and that some of these changes could be carried on and propagated throughout different passages of the organoids. Organoid lines can be established from patient biopsies acquired before and after disease recurrence and are interchangeable with orthotopic xenografts. Notably, organoid lines often retain parental tumor heterogeneity and exhibit a spectrum of genomic changes that are consistent with tumor evolution in culture.
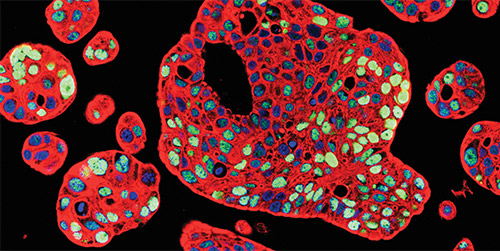
Immunostaining for p53 (green) and cytokeratin 8 (red) with nuclear counterstain (blue) in patient-derived human bladder tumor organoids (Courtesy of Dr. Michael M. Shen)
“Importantly, the organoids replicate some of the morphologic changes in the tumor as well,” says Dr. Lenis. “Dr. Shen and his team showed that responses to therapy that were observed in the patient could be mimicked in the organoid as well. These are powerful tools to study tumor biology and mechanisms of response and resistance.”
According to Dr. Lenis, the organoid model is complementary to in vivo models. “The organoid model demonstrates some advantages over a genetically engineered mouse model, primarily because it is much easier to establish and create an organoid versus establishing a tumor model in a mouse. The timeline is much quicker to look at certain drug response studies. And ultimately, these are patient derived tumors and so, there are some advantages to using human tissue as well. On the other hand, organoid models are more limited in studying the interaction between the tumor and the microenvironment and that’s where in vivo models, including mouse models, are very useful.”
Dr. Shen and his team showed that responses to therapy that were observed in the patient could be mimicked in the organoid as well. These are powerful tools to study tumor biology and mechanisms of response and resistance.
— Dr. Andrew Lenis
One of Dr. Lenis’s goals is to create a personalized therapeutic strategy for each patient. “The timeline to be able to create an organoid and treat it and then look for a response is on the order of a few weeks,” says Dr. Lenis. “Essentially, we establish organoids from clinical trial patients and evaluate the response of the organoid to treatment concurrently while the patient is undergoing treatment. This process enables us to gain more insight into the mechanisms of response and resistance. Ultimately, we hope to establish an organoid from a patient to help us determine the best treatment for that patient.”
“We are still in the discovery phase of using organoids to understand mechanisms,” continues Dr. Lenis. “By understanding the mechanism, we can determine where to intervene and where the therapeutic vulnerabilities are in some of these processes. One example is demonstrated in the 2018 Cell paper. In that initial biobank of bladder cancer samples, there was an interesting finding where a subset of the tumors displayed plasticity in which the tumors initially exhibit a luminal molecular subtype. In culture, the organoids demonstrated this plasticity and would transition to a more basal squamous phenotype. That is relevant because luminal tumors and basal squamous tumors may behave differently and have different responses to therapy.”
“We have been studying that process of plasticity in organoids and different mechanisms to limit that process,” continues Dr. Lenis. “By limiting that plasticity, we may be able to impact the progression event to reduce the chance that these patients progress to more invasive or metastatic disease. There is a complex interplay and interaction with the microenvironment in the stroma surrounding the tumor. What we're essentially trying to do is understand how these components interact to drive progression and then understand where to short circuit the process.”
Understanding Lymph Node Metastasis
Dr. Lenis explains that traditionally the lymphatics have been regarded as passive channels for lymph fluid or conduits for the spread of cancer. Over the years, studies of a number of malignancies have shown that the lymphatic microenvironment is a complex group of cells. “They're very heterogeneous,” says Dr. Lenis. “It is an environment consisting of lymphatic endothelial cells, stromal components, and tumor cells that involve a very complex interplay among these components. The lymphatic endothelial cells can actually contribute to and propagate metastasis as they secrete factors that cause tumor cells to home to the area. There are also different processes that allow lymphatic channels to expand and receive tumor deposits.”
Lymph node metastasis is another area of research for Dr. Lenis and his colleagues in the Shen Lab. “Patients with bladder cancer often present with or develop lymph node metastasis in the course of their disease,” says Dr. Lenis. “About one-third of patients who are found to have lymph node metastasis at the time of surgery will respond successfully to combinations of chemotherapy and surgery. Unfortunately, some patients do not respond and ultimately die of their disease. What drives this process of lymph node metastasis is not well understood, nor has it been studied sufficiently within bladder cancer. Currently there are no targeted therapies for patients who have lymph node metastasis. One of our goals is to study this process to try to develop more targeted therapies.”
Supporting Translational Science
Dr. Lenis encourages building translational aims into clinical trials. “We offer many clinical trials in every area of cancer, and it is extremely important that we obtain material, including blood, urine, and tissue, for translational investigation with these trials,” he says. “Organoids would be created from patients enrolled in the clinical trial, and both the patient and the organoid would be treated with the same drug to answer whether the responses observed with the organoids in the laboratory resemble the patient responses to the same drug. It’s not enough to know if a drug works or not. We also need to know why it’s working and why it’s not working in others to help us advance the field.”




