“Precision medicine and tumor sequencing have the potential to change the course of treatment for children with advanced or metastatic brain tumors,” says Jeffrey P. Greenfield, MD, PhD, a pediatric neurosurgeon in the Department of Neurological Surgery at NewYork-Presbyterian/Weill Cornell Medical Center, and Co-Founder and Co-Director of the Children’s Brain Tumor Project at Weill Cornell Medicine along with Mark M. Souweidane, MD, Director of Pediatric Neurological Surgery at NewYork-Presbyterian/Weill Cornell.

Dr. Jeffrey Greenfield

Dr. Mark Souweidane
Through the Children’s Brain Tumor Project, Dr. Greenfield and Dr. Souweidane focus on novel approaches and innovative care for children with rare and inoperable brain tumors. Dr. Greenfield is also the first scientific director of the Children’s Brain Tumor Project and leads its neuro-oncology research laboratory. He became one of the earliest adopters of precision medicine approaches for children with brain tumors and is among the leading advocates for the advancement and wider application of biomarker-driven targeted therapies.
“When one discusses adult brain tumors as a comparison, there are only a few types of tumors that are generally referred to,” says Dr. Greenfield. “In the pediatric landscape, however, there are literally 30 or 40 and some say as high as 70 or 80 different types or subtypes of brain tumors. The vast majority of children with these tumors have not seen a significant change in our ability to treat them effectively. The current standard of care has failed to significantly improve clinical outcomes for many of these patients, particularly those with high-grade, inoperable, recurrent, and/or metastatic tumors.”
“The evolution of precision medicine is staggering to think about,” continues Dr. Greenfield. “As a global term, precision medicine has taken on a number of iterations over the last 20 years. It started out with the idea that you sequence the DNA derived from a tumor cell to acquire the signature of what drives that tumor. This has since transitioned to where we are now thinking about the approach as precision therapeutics. How can we use the information that has been derived from the tumor to effect meaningful and intelligent decisions regarding what is likely to work on that tumor?”
As one example, Dr. Greenfield points to medulloblastoma, the most common type of brain tumor in children. “These tumors all look fairly similar on MRI and under a microscope,” he says. “But if you examine the actual signature of the DNA within those tumors, that analysis reveals a broad distinction between several different classes that are defined by their molecular signature. That molecular signature now defines what type of medication may treat that tumor more successfully. And even more powerfully, it may show which children may not need the most toxic types of treatment. Genomic sequencing offers a spectrum of information for designing therapies and also for whittling back therapies that may not be necessary.”
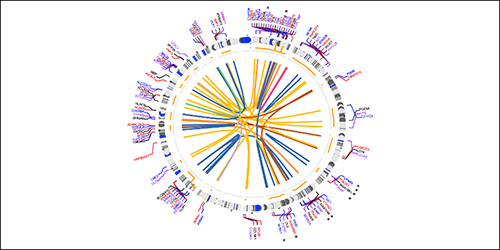
Fusion detected from the pediatric brain tumors resected by the Department of Neurosurgery at NewYork-Presbyterian/
Dr. Greenfield and colleagues in the Departments of Neurological Surgery and Pathology and Laboratory Medicine at Weill Cornell Medicine recently led a study to explore the clinical value of incorporating high-throughput assays with broad genomic assessment – whole-exome sequencing, RNA sequencing, and methylation array profiling – as part of the workup of pediatric tumors. Paired tumor and normal control specimens from 53 pediatric patients with central nervous system (CNS) tumors underwent whole-exome sequencing; a subset of patients also underwent RNA-sequencing (n = 28) and/or methylation array analysis (n = 27).
In their findings, which were published in the March 10, 2022, issue of Neuro-Oncology Advances, Dr. Greenfield and the research team noted that over a heterogeneous set of pediatric tumors, RNA-sequencing and methylation profiling frequently yielded clinically relevant information, while whole-exome sequencing demonstrated clinically relevant added value primarily via copy number assessment. According to the investigators, this study is one of the first to examine the combined clinical utility of whole-exome sequencing, RNA-sequencing, and methylation profiling within the framework of clinical management of pediatric patients with CNS tumors.
Translating Research in Real Time
At NewYork-Presbyterian/Weill Cornell, when a child is diagnosed with a brain tumor that requires surgery, a precision medicine algorithm is automatically initiated. “Tissue from different parts of the tumor, along with cerebral spinal fluid and a blood sample, are rapidly collected and taken both to the pathology laboratory and also to the Children’s Brain Tumor Project lab,” says Dr. Greenfield. “There, we isolate DNA and RNA to do the analyses. Concurrently, we culture those cells to implant into a mouse model, which allows us to grow the tumors in a brain environment. Based upon what the DNA analyses suggest, high throughput drug screening enables us to test hundreds of different drugs against the patient’s actual tumor.”
“We’ve progressed from this type of molecular analysis being purely a scientific endeavor into one in which we are now making substantial therapeutic and medication decisions. We are also better able to give prognoses to families and give more information to the treating oncologist.” – Dr. Jeffrey Greenfield
“We’re growing the child’s exact tumor in replicate and in triplicate in the mouse model and actually reproducing their disease course and, in some cases, testing these drugs against cells that have been radiated,” further explains Dr. Greenfield. “There is no point in treating cells that have not been radiated when the actual tumor has. While we’re in the laboratory, we can say, ‘At this point, this is what we think is going to be most active against this child’s tumor,’ and intervene during the child’s disease course. This is a very new and exciting part of our program. We are trying to duplicate in the laboratory what the child is going through in the clinic.”
Moving Precision Medicine into the Mainstream
According to Dr. Greenfield, one of the ongoing challenges of precision medicine is to accelerate the time between removing the tumor and the child’s recovery from surgery and the next phase of their treatment based on what is learned in the lab. He believes a number of hurdles must be overcome before the precision medicine approach to pediatric brain tumors becomes mainstream.
“We’ve progressed from this type of molecular analysis being purely a scientific endeavor into one in which we are now making substantial therapeutic and medication decisions,” says Dr. Greenfield. “We are also better able to give prognoses to families and give more information to the treating oncologist. But for precision medicine to enter into the mainstream of treatment and become a game changer, a number of things need to occur.”
“Molecular sequencing and analysis is not offered at every institution,” continues Dr. Greenfield. “Therefore, first, precision medicine needs to be considered as part of the standard of care from the very beginning of the cancer diagnosis. Unfortunately, standard of care, which is often unsuccessful, also changes the biology of the tumor. However, if DNA from the patient’s tumor is obtained early on in order to conduct analysis of a de novo tumor, we would get radically different information on how to treat that tumor rather than analyzing a tumor that has been already been exposed to radiation or chemotherapy, often multiple times. While we are starting to make headway, the thinking that this should be considered upfront has not yet reached mainstream consciousness. I spend a lot of time thinking about this because we are dealing with children who don’t have a lot of time.”



