The Pediatric Cancer Foundation Developmental Therapeutics Program at Columbia was established in 2006 with the singular goal of offering care to patients whose cancer recurs or does not respond to treatment. “According to CureSearch, 85 percent of children diagnosed with cancer will be cured, but that still leaves too many children for whom our standard therapies are not succeeding,” says Luca Szalontay, MD, a pediatric neuro-oncologist in the Division of Pediatric Hematology, Oncology, and Stem Cell Transplantation at NewYork-Presbyterian Morgan Stanley Children’s Hospital. “The diverse portfolio of medicines that is being evaluated in our clinical trials gives our patients access to many therapies that are not available elsewhere. These include new cytotoxic drugs, targeted biologic agents, immunotherapies, oncolytic viruses that are specifically engineered to attack cancer, and locally applied injectable therapies.”
The clinical leadership of the Developmental Therapeutics Program includes Dr. Szalontay, who directs the brain/central nervous system tumors section; Darrell Yamashiro, MD, Chief of the Division of Hematology, Oncology and Stem Cell Transplantation, NewYork-Presbyterian Morgan Stanley Children’s Hospital, who leads the solid tumors section; Nobuko Hijiya, MD, Section Head of Oncology at NewYork-Presbyterian Morgan Stanley Children’s Hospital and the program’s Medical Director, who spearheads efforts in hematologic malignancies; and Jennifer Oberg, EdD, MA, who serves as Director of Research. The team oversees some 36 clinical trials of novel anti-cancer agents for patients with cancers that are refractory or recur after treatment, including:
- Liquid tumors – acute lymphocytic leukemia (ALL), acute myeloblastic leukemia (AML), chronic myeloid leukemia (CML), and Hodgkin’s and non-Hodgkin’s lymphoma
- Brain tumors
- Extracranial solid tumors
“Often these drugs were already tested in adult clinical trials,” says Dr. Szalontay. “We work very closely with Columbia’s adult medical oncologists to better serve the adolescent and young adult populations. Their clinical studies are available for patients over 18 years of age and sometimes even younger. We are seeking opportunities to open studies in collaboration so that perhaps the age limit can be lowered and our younger patients will have more opportunities to participate in them.”
“Very rarely are the drugs specifically designed for pediatric disease and, in those cases, we offer first-in-children phase 1 and 2 trials,” continues Dr. Szalontay. “Our program is focusing on making these drugs available for children in these early phase clinical trials, either through different consortiums or sometimes directly working with pharmaceutical companies.”
“In the world of early-stage clinical trials, there are usually only a few slots available for patients,” notes Dr. Hijiya. “Then you have to pause to evaluate toxicity data, so the number of available studies changes even on a daily basis. Since the program’s inception, over 300 patients have been enrolled in developmental therapeutics managed studies, with about 15 new patients a year entering these studies.”
According to Dr. Szalontay, there are a number of ways through which the Developmental Therapeutics Program offers these trials, including the Pediatric Early Phase Clinical Trials Network (PEP-CTN), formerly known as the Children’s Oncology Group Phase 1 Consortium. “Columbia is one of only 21 institutions in North America offering early drug development trials,” says Dr. Szalontay. “We also participate in the National Cancer Institute Pediatric MATCH [Molecular Analysis for Therapy Choice] trial, which is designed to look for specific mutations in tumors that are centrally tested and then the genetic alterations are matched with specific targeted agents. There are about 10 of those trials available and open.”
The Columbia Developmental Therapeutics Program is also a member of the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) consortium. Comprised of 31 institutions, TACL seeks to develop innovative therapies for currently incurable leukemia and lymphoma. “Their approach is to integrate translational laboratory research with early phase clinical trials to speed the progress of new therapy development for children with leukemia and lymphoma,” says Dr. Hijiya.
Columbia Developmental Therapeutics Program – Clinical Trials Open for Enrollment
Following are some of the studies at Columbia that are actively recruiting patients:
Pediatric Early Phase Clinical Trials Network
- Phase 1/2 study of tegavivint in children, adolescents, and young adults with recurrent or refractory solid tumors, including lymphomas and desmoid tumors
- Phase 1/2 study of elimusertib in pediatric patients with relapsed or refractory solid tumors
- Non-randomized, open-label, multicenter, phase 1/2 study of PI3K inhibitor copanlisib in pediatric patients with relapsed/refractory solid tumors or lymphoma
- Phase 1/2 study to evaluate palbociclib (Ibrance) in combination with irinotecan and temozolomide in pediatric patients with recurrent or refractory solid tumor
- Phase 1, open-label, dose-escalation trial of CD33xCD3 bispecific antibody in pediatric patients with relapsed or refractory acute myeloid leukemia
- Open-label, multicenter phase 1/2 study of surufatinib in combination with gemcitabine in pediatric, adolescent, and young adult patients with recurrent or refractory solid tumors
Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL)
- Pilot study of vincristine sulfate liposome injection (Marqibo®) in combination with UK ALL R3 induction chemotherapy for children, adolescents, and young adults with relapse of acute lymphoblastic leukemia
- Phase 1 trial of temsirolimus in combination with etoposide and cyclophosphamide in children with relapsed acute lymphoblastic leukemia and non-Hodgkin’s lymphoma
- Phase 1/2 study of PO ixazomib in combination with chemotherapy for childhood relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma
Columbia Developmental Therapeutics Program
Current Clinical Trials Portfolio
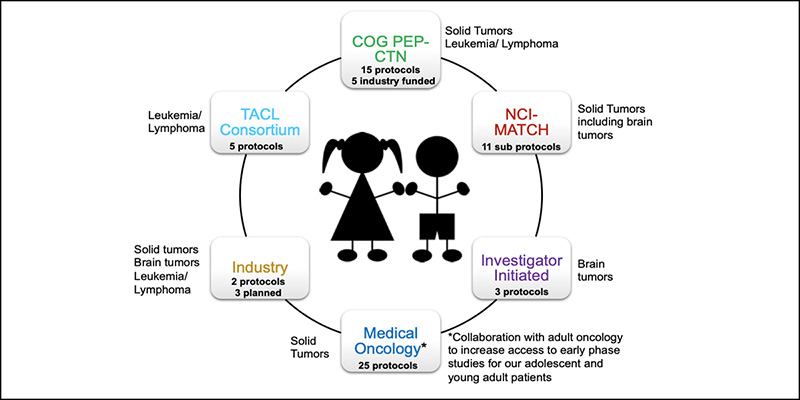
Dr. Hijiya and Dr. Szalontay point out that some of the drugs they are evaluating are disease agnostic. Rather than focused on one type of cancer, the drug may be evaluated in a basket trial, which involves a larger cohort of patients representing different tumors but undergoing treatment with the same novel medication that targets a mutation that is common to the different cancers. “Instead of looking for specific types of tumors, these studies look for specific mutations in any kind of tumor,” says Dr. Hijiya. “When we define the mutations, we can then offer the medication to patients with these mutations.”
“Our faculty bring a range of expertise in different types and stages of cancer,” continues Dr. Hijiya. “For example, Dr. Szalontay is currently leading a drug delivery innovation program for pediatric brain tumors with radiation oncologist Dr. Cheng-Chia Wu. They are testing different methods to overcome the blood-brain barrier, including one that uses convection-enhanced delivery for DIPG and another that utilizes focused ultrasound for progressive DIPG. Collaborating with Dr. Szalontay and other physicians with such a broad range of expertise enables us to explore novel therapeutics in great depth.”
How Molecular Drivers Inform Potential Therapies
In addition, Dr. Jennifer Oberg leads the Precision in Pediatric Sequencing (PIPseq) program that seeks to identify the molecular drivers of each patient’s cancer through clinical grade whole exome and transcriptome sequencing and then to use this information to make more personalized treatment recommendations, including new, biologically targeted investigational agents. The PIPseq program focuses on high-risk pediatric patients with relapsed/refractory disease with a predicted survival of <50 percent and patients newly diagnosed with unusual tumors that have no standard of care therapy.
“We are just starting to understand pediatric tumors on a molecular level and that these tumors are very heterogeneous,” says Dr. Szalontay. “We now realize that we need to use combination therapies and very specifically targeted therapies to be successful in these diseases. By prospectively sequencing these cancers and creating personalized avatars to determine whether the gene mutations identified by PIPseq contribute to the cancer’s development, we can then use the results to make clinical decisions for our patients. It’s not going to happen overnight, but I believe we are on the right path.”



