Scientific collaboration plays an integral role in the craniofacial care that patients receive at NewYork-Presbyterian Komansky Children’s Hospital. Caitlin Hoffman, MD, Co-Director of the Multidisciplinary Craniofacial Program at NewYork-Presbyterian Komansky Children’s Hospital, and Thomas Imahiyerobo, MD, of Pediatric Plastic Craniofacial Surgery, are collaborating with both the Ross Laboratory at the Weill Cornell Medicine Feil Family Brain & Mind Research Institute and the Greenblatt Laboratory at Weill Cornell Medical College to conduct genomic research on craniosynostosis.
“There is a dramatic advantage for families who are able to obtain preemptive and proactive knowledge. The earlier we have access to [a child’s] genetic footprint, the more tools we have to guide and serve them. The older these patients get, the more options get taken off table,” says Dr. Hoffman.
Genetic Mosaicism Studies
In their collaboration with Margaret Elizabeth Ross, MD, PhD, patients undergoing craniosynostosis surgery with Drs. Hoffman and Imahiyerobo consent to provide small skin and blood samples for study. The Ross Lab then grows and magnifies fibroblasts in culture and extracts DNA for whole genome sequencing (WGS), which is not yet standard clinical practice.
“In many cases of craniosynostosis, a clear genetic cause is not found on routine testing. Since we have DNA and fibroblasts, we can go several steps beyond traditional methods to use very deep sequencing and advanced computational tools to look for changes in genes that Dr. Greenblatt’s studies indicate are important for suture closure,” says Dr. Ross.
Genomic data visualization
The possibility that mosaic mutations are contributing to craniosynostosis represents a novel insight in the field and Dr. Ross plans to publish the findings soon, in collaboration with Drs. Hoffman and Greenblatt.
“Often, we do not find a clear genetic explanation for the condition. One possibility is that early in embryogenesis, there could have been a mutation in some, but not all cells of the developing embryo—hence the term mosaic. We are looking for variants within the genes that are associated with craniosynostosis by using new computational tools to detect mosaicism from more typical [WGS], but that compares well with the sensitivity of deep sequencing. In the future, this method could be more widely available to families. Genetic assessment that includes looking at mosaicism can give us more accurate knowledge of recurrence risk in family planning and also help to better assess risks that may lie ahead for a child,” says Dr. Ross.
“In many cases of craniosynostosis, a clear genetic cause is not found on routine testing. Since we have DNA and fibroblasts, we can go several steps beyond traditional methods to use very deep sequencing and advanced computational tools to look for changes in genes that Dr. Greenblatt’s studies indicate are important for suture closure.” — Dr. M. Elizabeth Ross
The genetic mosaicism study results will ultimately help Dr. Hoffman to better counsel families on potential treatment options for craniosynostosis. “We are focusing on timing of treatment, method of treatment, and how this will impact outcomes,” says Dr. Hoffman.
By seeking to better understand the genetic footprint and where a patient might fall on the germline versus mosaic spectrum, the researchers are helping to pinpoint the optimal timing of craniosynostosis treatment and uncover which patients might benefit from a minimally invasive endoscopic approach versus an open surgical approach.
“I envision that if we can understand the genetic profile, including the mosaic spectrum of a patient, then we can individualize an optimal treatment approach and monitor patients in a much more individual way. We are using precision medicine and applying it to cranio-genetic realm,” says Dr. Hoffman.
Stem Cell Discoveries
In their collaboration with Matthew Greenblatt, MD, PhD, Drs. Hoffman and Imahiyerobo provide bone specimens to the Greenblatt Lab to create mouse models with craniosynostosis. The researchers are using animal models to investigate potential medical treatments that might be complementary to surgery.
“This research enhances clinical care because we have surgeons like Dr. Hoffman who not only provide the standard of care, but are creating tomorrow’s standard of care for these patients,” says Dr. Greenblatt.
In 2018, Dr. Greenblatt and colleagues reported in Nature the discovery of a population of periosteal stem cells on the outer surface of bone that are responsible for growing and healing bone. “If these stem cells are so important, we thought that a lot of disorders of the skull should be caused by problems in this cell,” says Dr. Greenblatt, who noted that one of the most important disorders of the skull is craniosynostosis.
To test whether these newly discovered periosteal stem cells were related to skull malformations, Dr. Greenblatt’s Lab deleted one of the most common genes known to cause craniosynostosis only in these new stem cells. As the researchers expected, creating genetic defects in periosteal stem cells in mice recreated the signature skull fusion seen in craniosynostosis, but what happened next, no one expected.
“To our surprise, instead of the stem cell being activated and causing overgrowth that leads to fusion of the skull, the stem cell died and another cell that no one had seen or expected emerged in its place,” says Dr. Greenblatt.
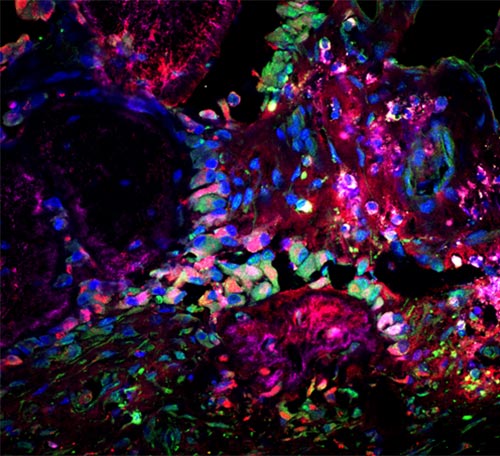
Microscopic image of a site of skull fusion in a patient specimen, showing bone-forming (magenta) and cartilage-forming (red) cells stained for a marker of a new stem cell population (green). (Source: Image courtesy of Seoyeon Bok, PhD, working in the lab of Matt Greenblatt, MD, PhD.)
In a series of experiments in mice and then patient specimens, Dr. Greenblatt’s Lab found a second stem cell that was attempting to compensate for the dead stem cell. “When there is a problem, the second stem cell senses it and tries to compensate but this compensation itself causes fusion,” says Dr. Greenblatt.
The Greenblatt Lab’s groundbreaking discovery of dysfunctional periosteal stem cells has profound implications for targeted medical therapy for the treatment of bone disorders like craniosynostosis. The researchers have a strong suspicion that there are other stem cells, just waiting to be discovered. They are continuing to pursue this research in collaboration with Dr. Hoffman.
“The [Greenblatt Lab] is on the precipice of a potential pharmaceutical approach. It is really exciting, groundbreaking mechanistic work. Up until our collaboration, we didn’t have any human models. We do three to four surgeries per week and by repurposing our discarded bone tissue and maximally utilizing blood samples, now we have novel discoveries,” says Dr. Hoffman.
“Now for the first time we know where to direct and target therapy in a way that may be complementary to what surgeons like Dr. Hoffman are doing,” adds Dr. Greenblatt, who is in the process of publishing the lab’s latest discoveries.
Advancing Clinical Care
Although it is understood that mosaicism contributes to structural birth defects, the challenge has been how to identify it, given the heavy reliance on exome sequencing. By using advanced genetic analysis, Dr. Ross’ Lab performs deep sequencing, which can look at every gene across the genome with a great depth of coverage.
“This gets us closer to a precision medicine approach so we can look very individually at a child and their family. We know more about the potential road ahead and how best to treat,” says Dr. Ross.
Similarly, Dr. Greenblatt’s Lab has pioneered advanced genetic analysis tools to uncover the genetic drivers that are involved with bone growth and sutures. “The technologies are specially developed in my group. We are pretty unique in terms of capabilities,” says Dr. Greenblatt, who is investigating the healing ability of periosteal stem cells in other places in the bone such as a fractured leg.
With their collaboration with Dr. Hoffman, Drs. Ross and Greenblatt are helping to translate genomic research into meaningful changes in clinical practice. The collaboration of neurosurgeons, neurogeneticists, and neurobiologists at the Multidisciplinary Craniofacial Program at NewYork-Presbyterian Komansky Children’s Hospital is helping to advance clinical care for craniosynostosis.
“I have not seen this individualized approach brought to this disease before. Both of these [studies] are exceptionally unique and on an international scale,” says Dr. Hoffman.



