Over the past two decades, remarkable strides have been made in the development of pediatric ventricular assist devices (VADs), including miniaturization and technological refinements, leading to a wider range of devices and options available to children. “Even in the last 10 years, it has been an amazing time to witness the growth in these devices and to be a part of their realization for children,” says Sabrina Pia Law, MD, Director of the Pediatric Ventricular Assist Device Program and member of the Program for Pediatric Cardiomyopathy, Heart Failure and Transplantation at NewYork-Presbyterian Morgan Stanley Children’s Hospital. Dr. Law is also Associate Professor of Pediatrics at Columbia.

Dr. Sabrina Law
Dr. Law’s passion for the field of mechanical support devices in children began during her Advanced Fellowship in Pediatric Heart Failure and Transplant at Texas Children’s Hospital, which, in 2011, was amongst the first hospitals in the country to implant a total artificial heart in a teenager. Not only was Dr. Law on hand to observe this landmark surgery, she notes this fellowship turned out to be a landmark year for her professionally. “I was captivated from that moment. I was so impressed by the innovation. At that time, while pediatric VAD was available, it wasn't as accessible as it is currently and typically only used as a bridge to transplant. In the decade since, VADs have evolved into destination therapy as treatment of choice for patients who are not transplant candidates or who prefer the VAD to transplant, and also as a bridge to recovery.”
Dr. Law, who joined the Division of Pediatric Cardiology at NewYork-Presbyterian Morgan Stanley Children’s Hospital in 2014, continues to pursue her dedication to evaluating and refining the use of VADs in children with acute decompensated heart failure. In her practice, she cares for children with end stage heart failure secondary to congenital or acquired cardiomyopathies, failed palliated or non-palliated terminal congenital heart disease, and acquired cardiac disorders. Since Dr. Law’s arrival, the number of VADs used as a bridge to transplantation or recovery at NewYork-Presbyterian Morgan Stanley Children’s Hospital has increased six-fold.
“When I first started at Columbia, we were doing about five VADs a year,” notes Dr. Law. “Now we are doing 30 to 35 devices a year, which includes patients who are implanted with a combination right ventricular assist device and left ventricular assist device and those patients who are switched from one type of device to another.”
With its broad range of medical, surgical, and transplant expertise, the Program for Pediatric Cardiomyopathy, Heart Failure and Transplantation supports the care of infants, children, and adolescents with the most challenging clinical circumstances. The team frequently cares for patients referred from other programs due to the complexity of the management and treatments that are needed to save their lives. “Oftentimes patients come to us extremely sick and in need of an immediate intervention, usually mechanical support,” says Dr. Law. “The percentage of patients who are on a VAD waiting for transplant nationwide is about 20 to 25 percent. For our program, the percentage is at least 50 percent because these kids are coming to us so advanced in their heart failure from other centers.”
“When I first started at Columbia, we were doing about five VADs a year. Now we are doing 30 to 35 devices a year, which includes patients who are implanted with a combination right ventricular assist device and left ventricular assist device and those patients who are switched from one type of device to another.” — Dr. Sabrina Law
Dr. Law emphasizes that a VAD should not be implanted in a patient who is severely ill or in a life-threatening situation. “We first stabilize the child based on the degree of their heart failure before taking them into the OR to do the implant.” At NewYork-Presbyterian Morgan Stanley Children’s Hospital this involves a meticulous evaluation performed by the team to assess factors such as the child’s anatomy and physiology, the stage of their heart failure, and if end-organ dysfunction is present.
“Similar to a transplant evaluation, multiple disciplines participate, including social work, nutrition, and psychiatry,” says Dr. Law. “We get to know the family because they need to understand what they’re walking into. We are very upfront about the many complications that can happen, especially in our smaller patients, so palliative care is also a significant part of the discussion.”
Elevating the Use of VADs in Children
As pediatric heart transplantation became more widespread, the use of VADs in pediatric populations became more common. However, VADs currently available for use in the pediatric population are limited to patients whose size can accommodate VADS designed for adults that can be modified for use in larger children. According to Dr. Law, the increased use of VADs in children has also prompted the pediatric clinical community to begin sharing their data and experience through networks, including the Pediatric Registry for Mechanical Circulatory Support (PediMACS) and the Advanced Cardiac Therapies Improving Outcomes Network (ACTION).
“The ACTION network began with a handful of physicians and now has over 1,000 healthcare providers from all over the world and includes family representatives,” says Dr. Law. “Through these collaborations and sharing of data, we are able to make changes to our practice in real time. These include three significant clinical practice changes over the past few years. Substituting a direct thrombin inhibitor as an alternate to heparin for anticoagulation greatly reduced the incidence of stroke following a Berlin Heart implantation. Additionally, the HeartMate 3 LVAD was approved for pediatrics as a direct result of the work of the ACTION network, resulting in FDA approval at the end of 2020. And, a few years ago, it was brought to light that continuous flow technology, which is used in the adult world for VADs, is much more physiologically and biomechanically suited for use in patients, so we shifted our attention to looking at miniaturized fully implantable, continuous flow pumps, one of which is now under investigation in the nationwide PumpKIN trial.”
A VAD for the Tiniest Patients
Dr. Law is particularly proud that NewYork-Presbyterian Morgan Stanley Children’s Hospital was selected as a vanguard site for the PumpKIN (Pumps for Kids, Infants, and Neonates) trial, a seven-center feasibility study of the investigational Infant Jarvik 2015 VAD in pediatric patients with heart failure who require mechanical circulatory support. The national trial, which began in October 2018, is expected to enroll 10 patients, with completion expected later this year. The PumpKIN program is the creation of the National Heart, Lung, and Blood Institute (NHLBI) in response to the lack of circulatory support devices that could be used for some of the smallest of children awaiting a transplant.
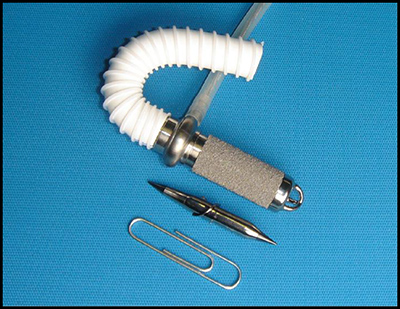
The Jarvik 2015 VAD is the size of a AA battery and adjusts the blood flow as the child grows. (Credit: Jarvik Heart)
“This fully implantable, continuous flow left ventricular assist device is the size of a AA battery and is designed for small children within a weight range of 8 to 30 kilograms,” says Dr. Law, who serves as Site Co-Principal Investigator for Columbia. “The trial is evaluating the safety of the device as both a bridge to transplant and to recovery. If successful, this will provide clinicians with an alternative to the current VADs such as the Berlin Heart, which is an effective therapy but can only be used as a bridge to transplant. The Berlin Heart is not completely implantable so children need to remain in the hospital to be carefully monitored until the time of transplant. With the Infant Jarvik VAD, children can potentially be discharged home.”
Dr. Law and her Columbia colleagues have so far implanted the Infant Jarvik VAD as a bridge to transplant in two children as part of the PumpKIN trial. “It has been astounding to see how this device is able to help the youngest of our patients,” notes Dr. Law. “The difference between the two VADs was particularly pronounced in one of our families in which we are treating two siblings who developed cardiomyopathy in infancy. One sibling received the Berlin Heart VAD at 4 months and the other received the Infant Jarvik VAD at about 10 months of age as part of the PumpKIN trial. The parents were astonished at the difference between their children in terms of rehabilitation and mobility and in meeting developmental milestones. As a child waits for transplant, one of the most important objectives is to get them as healthy and as strong as possible before they go into the OR. The parents felt that the sibling who had the Infant Jarvik VAD implanted was much further along in that he was able to be weaned and had learned to walk with the Infant Jarvik VAD, which was remarkable to witness. He then had his transplant at 18 months.”
Dr. Law emphasizes that prior to the PumpKIN trial opening in late 2018, the device had been in development for about a decade undergoing many iterations involving the FDA, the NIH, and the developers. “There were several pumps that underwent testing in animal models. Every time the animal studies weren’t reassuring, the pumps were refined in their design,” says Dr. Law. “To follow that trajectory as a clinician and then to see one come to fruition in the clinical trial has been very exciting.”
“The 50cc total artificial heart, while it is an old device, is still an important FDA-approved option for children 10 years of age or older and weighing at least 20 kilos,” notes Dr. Law. “The total artificial heart is also incredible because it provides biventricular support. Oftentimes our patients require both a left ventricular assist device and a right ventricular assist device. The total artificial heart gives support with one device in a tiny chest that has limited real estate to accommodate two VAD devices. Most recently, the Berlin Heart technology was updated with a much smaller, much more portable mini driver with a battery life of six to eight hours as compared to 30 minutes for the older model, which allows for much more mobility for our young patients while awaiting transplant. Here at Columbia, we are fully certified, trained, and equipped to perform the total artificial heart procedure.”
Ongoing research by Dr. Law, her colleagues at Columbia and at medical centers around the world continues to expand the understanding of ventricular assist devices in pediatrics, provide guidelines for their current use, and raise new questions as VADs become more relevant in the care of pediatric heart disease.



