Results of a phase 2 clinical trial demonstrate the potential of targeted therapy in combination with chemotherapy to treat patients with uterine leiomyosarcoma, a rare, aggressive form of cancer that affects some 2,500 women every year in the United States.
The research was led by Gary K. Schwartz, MD, Deputy Director of the Herbert Irving Comprehensive Cancer Center, Chief of the Division of Hematology and Oncology at NewYork-Presbyterian/Columbia University Irving Medical Center, and a co-investigator in the clinical trial, and Matthew Ingham, MD, Assistant Professor of Medicine in the Division of Hematology and Oncology at Columbia and the trial’s lead investigator. The drug combination resulted in durable responses with a progression-free survival of almost seven months in a group of patients who progressed after front-line chemotherapy.

Dr. Gary Schwartz

Dr. Matthew Ingham
A malignant connective tissue tumor of smooth muscle origin, uterine leiomyosarcoma represents approximately 80 percent of uterine sarcomas and is often detected during routine gynecological surgery done for suspected fibroids. Most patients show no symptoms before diagnosis, but if the cancer has metastasized, the prognosis is extremely poor.
Later-line treatment options for uterine leiomyosarcoma provide limited benefit and are usually quite toxic. Only about 10 percent of patients respond, and even then, progression-free survival typically lasts for three to four months at the most. The clinical trial aimed to test a novel therapy for uterine leiomyosarcoma – olaparib – a PARP inhibitor originally developed for individuals with BRCA1 or BRCA2 mutations.
“Uterine leiomyosarcoma is a deadly disease. In the metastatic setting, it is incurable, and even when resected, recurrences are high,” says Dr. Schwartz. “Chemotherapy has been tried with only a very modest effect on improving survival. So our idea was to use precision medicine to define a subset of women who would benefit from a PARP inhibitor.”
The study involved 22 patients who received olaparib along with a low dose of temozolomide, a chemotherapy drug. Six patients (27 percent) had a durable response that lasted an average of 12 months. Four patients remain on treatment after two years. Side effects of the therapy, which mostly included low blood counts, were tolerable without any serious complications. Based on these promising initial results, the team received support from the National Cancer Institute to launch a large, randomized phase 3 clinical trial to test this novel drug combination against the current standard of care.
Olaparib works by blocking PARP, a family of proteins that help repair damaged DNA. Harmful mutations in the BRCA1 or BRCA2 genes lead to faulty DNA repair, and further inhibiting this process with olaparib can cause cancer cells that carry a BRCA mutation to die.
We think that this will eventually become a new standard of care for patients with uterine leiomyosarcoma and will have a profound effect for women with the disease for which there are no highly effective therapies.
— Dr. Gary Schwartz
However, other kinds of tumors can exhibit “BRCAness,” a more general term that refers to this same kind of defect in DNA repair caused by mutations other than BRCA1/2. Preclinical research from investigators at the Herbert Irving Comprehensive Cancer Center and other institutions identified the existence of BRCAness in uterine leiomyosarcoma, as well as the potential efficacy of combining PARP inhibitors with chemotherapy in this disease.
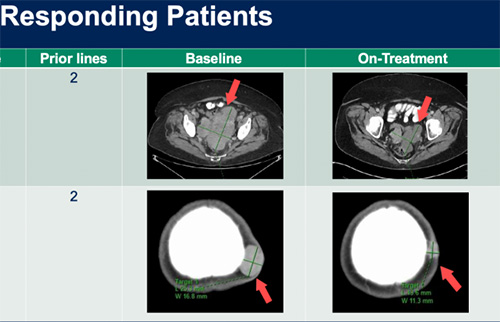
Representative images from a subset of patients, with two prior lines of treatment, achieving partial response on the study. Top: A patient who experienced reduction in size of a large pelvic mass; Bottom: A patient who had significant shrinkage of a scalp lesion among other sites of metastatic disease (Courtesy of Dr. Matthew Ingham)
“This is a good example of research that went all the way from the laboratory to the clinic at Columbia,” says Dr. Ingham. “Two avenues of research came together at the same time. First, uterine leiomyosarcoma was found to have BRCAness. Second, the PARP-inhibitor based combination was shown to be very effective in preclinical research using uterine leiomyosarcoma cell lines in Dr. Schwartz’s lab.”
The Columbia team also analyzed tumor biopsy specimens from patients participating in this study for genomic signatures of BRCAness that may predict which patients would benefit from taking olaparib. The results of this research, done in collaboration with Dana Farber Cancer Institute, identified a novel biomarker called a RAD51 assay that appears promising for identifying uterine leiomyosarcoma tumors with BRCA-like features that are more likely to respond to this therapy. This test can be done rapidly on tumor tissue and will be further evaluated in the upcoming clinical trial.
Dr. Schwartz and Dr. Ingham, along with Sminu Bose, MD, reviewed a range of novel therapeutics in the treatment of uterine sarcoma in the April 2022 issue of the American Society of Clinical Oncology Educational Book. The publication focuses on the most common histologies, including leiomyosarcoma, high- and low-grade endometrial stromal sarcoma, adenosarcoma, and other rare uterine sarcoma subtypes that harbor potentially actionable genomic alterations.





