Endocrinologists at NewYork-Presbyterian/Weill Cornell Medicine are advancing the understanding of molecular and cellular biology to improve the treatment of diabetes and other endocrinologic conditions. Recently, Laura Alonso, MD, Chief of the Division of Endocrinology, Diabetes and Metabolism at NewYork-Presbyterian/Weill Cornell Medicine, led a team that discovered a novel mechanism to stimulate growth of pancreatic beta cells in a preclinical model of type 2 diabetes.
In their study, published in the Journal of Clinical Investigation, Dr. Alonso and colleagues described how activation of a pathway to promote cell division not only increased the population of insulin-producing pancreatic beta cells, but also enhanced the cells’ functionality.
“For many of my patients, the root cause of their diabetes is that they don’t have enough pancreatic beta cells or their pancreatic beta cells aren’t working well enough,” notes Dr. Alonso, who has been studying pancreatic beta cells – the body’s sole source of insulin – since she assumed her first faculty position at the University of Pittsburgh in 2005. At that time, she and her colleagues focused on a mouse model of diabetes that lacks IRS2, a protein that enables insulin to transmit its signal for cells to absorb blood sugar. “The mice that don’t have IRS2 get a type of diabetes that is really similar to human diabetes because the whole body doesn’t respond to insulin correctly,” she says.
Leveraging the Interplay of IRS2, CDK4, and Cyclin D2
In their recently reported study, Dr. Alonso’s team zeroed in on CDK4, a protein that stimulates cell division, in IRS2-deficient mice. “CDK4 has long been known to be important in pancreatic beta cells,” she explains. “In mice, if you remove the CDK4 gene, those mice can’t make functional CDK4 protein, and they get diabetes. And the root cause of their diabetes is that they don’t have enough beta cells.” Inspired by rat studies showing a correlation between increased blood sugar and stimulation of pancreatic beta cell production, her team tested whether that was also true in mice, “so that we could then use all of the many genetic tools that are available in mice that aren’t available in rats. When we increased blood sugar in the mice, they did indeed make more beta cells, and we determined that was in part due to an increase in cyclin D2, a protein that partners with CDK4 to drive cell division.”
Introducing an active form of CDK4 normalized blood sugar levels in the previously diabetic mice, Dr. Alonso’s team reported. Additionally, the beta cells in these mice were more abundant than in untreated mice, and looked much healthier. The researchers concluded that boosting the activity of CDK4 greatly expanded the population of beta cells packed with insulin, supporting the concept that beta cell mass can be increased without compromising function.
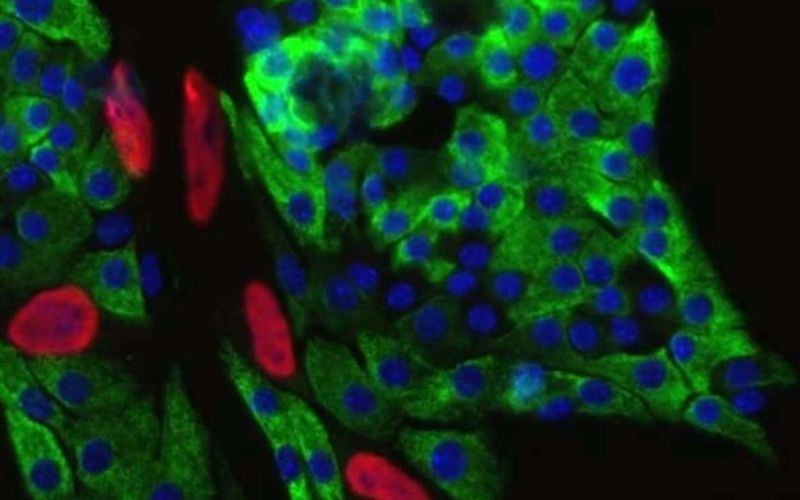
Immunofluorescence imaging of insulin-producing pancreatic beta cells from healthy mice. Green: insulin. Blue: cell nucleus. Red: a tag that indicates cell division. (Credit: Alonso Lab)
The “Yin and Yang” of Cell Division and Differentiation
Dr. Alonso and her colleagues also reported that the beta cells in the treated mice remained differentiated, in that they retained their specialized ability to make and secrete insulin. “Most people think of a fully differentiated cell that is specialized in one thing as being post-mitotic, meaning it can no longer enter the cell cycle and divide into two cells. Beta cells were thought for a long time to fall into that category,” she comments. “It’s become clear that fully specialized or fully differentiated beta cells can actually divide. But there is some evidence that when beta cells enter the cell cycle and start to divide, they become ‘de-differentiated,’ at least temporarily. De-differentiation may be a normal part of what happens to beta cells as they divide. Hopefully, the two daughter cells that come from that original cell will regain the ability to do their job.”
Exploring the so-called “yin and yang” of cell division and differentiation status “goes back to my very first baby steps as a scientist in diabetes,” Dr. Alonso relates. “I knew that when you raise the blood sugar in the mouse, it causes beta cells to enter the cell cycle, but I didn’t know why. I remain really interested in understanding the triggers that cause beta cells in the pancreas of a live mouse or, ideally, a human being, to decide to replicate and expand in number because that is sort of the holy grail: to essentially cure types of diabetes that are due to not enough insulin.”
Paving the Way to Future Therapeutic Benefits
Notably, CDK4 is not, in itself, a viable therapeutic target because its stimulation of cellular proliferation may increase the risk of cancer. Nevertheless, Dr. Alonso is optimistic that studying the canonical cell replication pathway, which includes CDK4, may eventually lead to a therapeutic breakthrough. She hopes to find a way to induce beta cells to replicate and increase in number without losing their function or causing cancer.
“Another holy grail is to find a way to activate the cell cycle in a regulated manner,” Dr. Alonso notes. “Most of the literature studying these cell cycle processes is in the cancer literature, and the goal of most of those studies is to stop them. As a beta cell biologist trying to activate the cell cycle safely, we’re sort of moving in the opposite direction of a lot of this literature.”
Understanding how these processes are regulated in human beta cells is an important ground floor for all kinds of exciting potential features.
— Dr. Laura Alonso
Dr. Alonso also notes that it is impossible to predict “when we’ll be able to help people’s own pancreas regrow their beta cells.” However, her team’s research may yield therapeutic benefits in the near term. She points out that stem cell-derived beta cells are already available as a cellular therapy. “Although that is not the same as my future goal of restoring the human body to its healthy state, it’s possible that our results will actually improve that type of therapy in the shorter term because that therapy already exists. Maybe this observation will help the process of making stem cells from an individual patient, so they don’t need to be immunosuppressed. Understanding how these processes are regulated in human beta cells is an important kind of ground floor for all kinds of exciting potential features,” she says.
Leveraging Institutional Expertise and Capabilities
Dr. Alonso cites the resources and in-house expertise at NewYork-Presbyterian/Weill Cornell Medicine as key to facilitating her team’s research. “Weill Cornell Medicine is completely amazing on so many different levels,” she says. That research prowess extends to the institution’s MD/PhD program. She points out that her study’s first author, Rachel Stamateris, was an MD/PhD student in her lab, where she received “critically important training to understand patient care and disease as well as the molecular biology and cell biology of how the body works.”
The Weill Center for Metabolic Health, which Dr. Alonso directs, includes several beta cell biologists as well as fat biologists and muscle biologists. “Right now we have a lot of basic wet lab research, and we’re also growing toward more clinical research and translational research as well,” she notes. “I am very interested in having the full range of research capability so that we can inform human clinical trials with the basic fundamental research going on in our departments here.”
That institutional expertise, combined with recent advances in diabetes therapeutics, makes Dr. Alonso hopeful for the future. “We are in such a better place now than we were 10 years ago in terms of medications for diabetes that don’t cause weight gain, medications that don’t just help with blood sugar, but also prevent heart attacks and stroke and keep the kidneys healthy,” she says. “And the technologies like insulin pumps, continuous glucose monitors, and insulin delivery algorithms are incredibly advanced compared to 10 years ago. So even though some of the future potential therapies seem like pie-in-the-sky science fiction, much more is possible if we follow good science.”



