In a randomized, controlled trial led in the U.S. by Brian G. DeRubertis, MD, FACS, Chief of the Division of Vascular & Endovascular Surgery at NewYork-Presbyterian/Weill Cornell Medicine, and Sahil A. Parikh, MD, Director of Endovascular Services at NewYork-Presbyterian/Columbia, an everolimus-eluting resorbable scaffold produced better results than angioplasty for patients with chronic limb-threatening ischemia (CLTI) from peripheral artery disease below the knee, also known as infrapopliteal artery disease. The results from the study were presented in October at the Transcatheter Cardiovascular Therapeutics (TCT) conference and were recently published in the New England Journal of Medicine.
Image of the everolimus-eluting resorbable scaffold. (Image from Varcoe RL et al. J Soc Cardiovasc Angiogr Interv. 2023;2(4):100964)
Drs. DeRubertis and Parikh had used an earlier version of the bioresorbable device in the coronary artery disease setting and felt it could be repurposed for patients with peripheral artery disease.
Patients with CLTI, an advanced stage of peripheral artery disease, experience rest pain, nonhealing wounds and even gangrene, putting them at high risk for amputation if they do not receive revascularization treatment. The condition disproportionately affects patients of color in the United States. Currently, the standard of care treatment for CLTI and infrapopliteal artery disease is angioplasty, but the procedure has several drawbacks, including a low patency rate.
“The one-year patency rate, or the chance of that artery is still open one year down the road, is somewhere between 30% and 50% historically, and that's quite poor,” says Dr. DeRubertis. “The problem is that a lot of these wounds will take between six and 12 months to heal, so there’s a large percentage of patients whose treated arteries are going to close back down within three months, or six months, or eight months and not be healed. They'll require a second procedure, with all its attendant risks.”
Some researchers have tried to repurpose coronary drug-eluting stents to address the shortcomings of angioplasty in CLTI below the knee. While drug-eluting metal stents improve the patency rate and help prevent the forming of scar tissue, they make it hard to perform future procedures.
Dr. DeRubertis says a drug-eluting resorbable scaffold appears to solve many of the problems of angioplasty and metal stents by improving patency through the poly-L-lactic acid scaffold structure, preventing scar tissue buildup and restenosis through the everolimus drug coating, and allowing for future interventions because the device is resorbable.
“The benefit of this device is that it has a mechanical and biologic effect, but then over about a one- to two-year period, it resorbs into the body, leaving a healed and healthy artery that could potentially even support a bypass if needed,” says Dr. DeRubertis.
The benefit of this device is that it has a mechanical and biologic effect, but then over about a one- to two-year period, it resorbs into the body, leaving a healed and healthy artery that could potentially even support a bypass if needed.
— Dr. Brian G. DeRubertis
Promising Results from the LIFE-BTK Trial
In the LIFE-BTK multicenter global trial, adults with CLTI and infrapopliteal artery stenosis or occlusion were randomized in a two-to-one fashion to receive treatment with the everolimus-eluting resorbable scaffold or with angioplasty. Treatment was performed on up to two lesions in the proximal two-thirds of the lower leg.
“We carefully selected sites globally that would allow us to recruit patients of all backgrounds,” says Dr. Parikh. “We endeavored to recruit minority patients and women, to the extent possible, by picking investigators who look like the patients we were trying to study. And we had sites in the Pacific Rim and Asia that also allowed us to enhance that population.”
The study’s primary efficacy endpoint was freedom at one year from the following events:
- amputation above the ankle of the target limb
- total occlusion of the target vessel
- clinically-driven revascularization of the target lesion
- binary restenosis of the target lesion
The primary safety endpoint was a composite of freedom from major adverse limb events at six months and perioperative death.
Overall, 173 patients received the drug-eluting resorbable scaffold, and 88 patients underwent angioplasty. At one year, 74% of patients in the scaffold group and 44% of patients in the angioplasty group met the study’s primary endpoint, an absolute difference of 30 percentage points. The study also showed that the drug-eluting resorbable scaffold was noninferior to angioplasty on the primary safety endpoint.
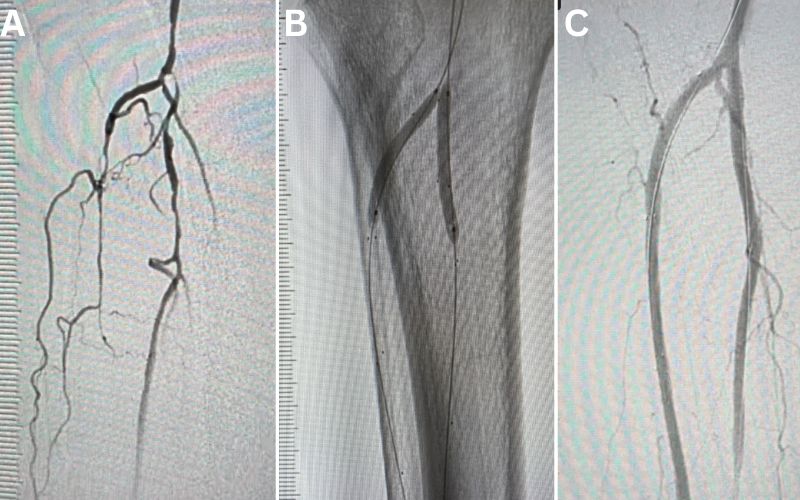
(A) Pre-procedural angiogram showing multifocal severe narrowing of several tibial arteries due to atherosclerotic plaque. (B) Deployment of drug-eluting resorbable scaffolds in two tibial arteries (anterior tibial and peroneal arteries). (C) Post-procedural completion angiogram showing restoration of normal tibial artery lumen with bioresorbable scaffold.
“The number needed to treat was only four, which is a very low number and reflects how robust the treatment effect of the scaffold was compared to angioplasty, the contemporary standard of care,” says Dr. Parikh.
The number needed to treat was only four, which is a very low number and reflects how robust the treatment effect of the scaffold was compared to angioplasty, the contemporary standard of care.
— Dr. Sahil A. Parikh
Breaking new ground for CLTI patients
The randomized LIFE-BTK trial is the first in more than a decade to show efficacy for a new device to treat CLTI and infrapopliteal artery disease. The findings are under review at the U.S. Food and Drug Administration, which must first approve the device before it could be used outside of a research setting.
“Hundreds of thousands of patients annually have chronic limb-threatening ischemia and roughly half of them will get an amputation without ever getting a proper vascular evaluation,” Dr. Parikh says. “If we can just get the message out that there are better options for these patients, and that we can potentially salvage those limbs, we could have a huge impact on the public health of this population.”
The trial also highlights the collaborative nature of work at NewYork-Presbyterian, with physicians in the cardiology and vascular surgery teams working together on this trial, rather than as competitors. “We’ve really done this work hand-in-hand [between departments and schools] and I think that’s a major distinction between us and other institutions in the country, if not the world,” Dr. Parikh says.





