Organoids Created from Patients’ Bladder Cancers Could Guide Treatment
Custom 3-D mini-tumors mimic individual patient’s cancer
Apr 5, 2018
New York
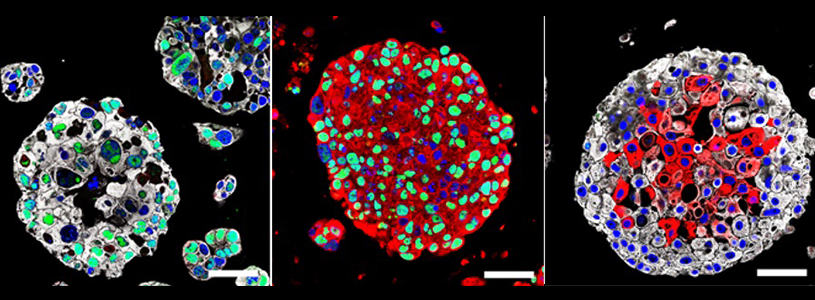
Columbia University Irving Medical Center (CUIMC) and NewYork-Presbyterian researchers have created patient-specific bladder cancer organoids that mimic many of the characteristics of actual tumors. The use of organoids, tiny 3-D spheres derived from a patient’s own tumor, may be useful in the future to guide treatment of patients.
The study was published today in the online edition of Cell.
In precision medicine, molecular profiling of an individual patient’s tumor is used to identify genetic mutations that drive that individual’s cancer. That knowledge may help physicians select the best drug to fight the cancer, but the analysis does not always predict how a patient will respond to specific therapies.
“The great advantage of organoids is that they are essentially avatars of a patient’s tumor,” said study leader Michael M. Shen, PhD, professor of medicine, genetics & development, urology, and systems biology at Columbia University Vagelos College of Physicians and Surgeons. “Having these personalized laboratory models, which we can make in a matter of weeks, will let us test multiple different drugs on the tumor and help us bring precision medicine to individuals with bladder cancer.”
Shen, who is also a member of NewYork-Presbyterian/CUIMC’s Herbert Irving Comprehensive Cancer Center, began developing bladder cancer organoids about four years ago. A major challenge in creating any type of organoid is determining the unique mixture of nutrients, growth factors, and tissue culture techniques that will transform patient tumor cells into miniature tumor organoids in a petri dish. The exact conditions can vary greatly from one type of cancer to another.
In the current study, organoids were made from the tumor cells of 22 patients with invasive bladder cancer.
The organoids, which can grow up to roughly 1 mm in diameter, appeared similar to the parent tumors and had many of the same molecular and genetic characteristics.
Importantly, the organoids developed genetic changes that occurred over time, a phenomenon known as clonal evolution. “Clonal evolution is a major driver of tumor progression and drug resistance. It’s remarkably difficult to model for a solid tumor,” said Shen. “With these organoids, we will be able to study how bladder tumors evolve and perhaps learn how to prevent tumors from becoming resistant to treatment.”
The researchers were able to make organoids from three patients both before and after treatment. “This offers a new way to study the molecular mechanisms associated with drug response and drug resistance,” said Shen.
Bladder cancer is the fifth most common cancer in the United States, yet it is one of the least understood because few animal models reflect the biology of the disease.
“The creation of bladder cancer organoids is an important advance in the field,” said study co-author James M. McKiernan, MD, the John K. Lattimer Professor of Urology and chair of urology at Columbia University Vagelos College of Physicians and Surgeons, and urologist-in-chief at NewYork-Presbyterian/Columbia. “This should greatly improve our understanding of the genomics of bladder cancer, how these tumors respond to drugs, and how they develop drug resistance. Ultimately, this may allow us to develop new therapies for the disease and predict an individual patient’s response to treatment.”
The researchers are planning to test the organoids’ predictive abilities in “co-clinical” trials, in which patients and their corresponding organoids are treated with the same drug. “This would establish whether organoids can be used to predict how an individual patient will respond to a specific therapy,” said Shen. “At present, it’s very difficult to know beforehand exactly which drugs may be most effective for a given patient.”
The current standard of care for patients with bladder cancer that has not invaded muscle is surgery to remove the tumor plus immunotherapy or chemotherapy. These tumors have a high recurrence rate, requiring repeat treatment. Some of these tumors progress to invade the bladder muscle, a form that is harder to treat and more lethal.
Patients with muscle-invasive cancer are typically advised to undergo bladder-removal surgery and chemotherapy. “Since bladder removal has such a profound effect on quality of life, most patients seek to avoid it,” said Shen. “We desperately need better, more targeted therapies for both types of bladder cancer.”
The paper is titled “Tumor evolution and drug response in patient-derived organoid models of bladder cancer.” The other authors are Suk Hyung Lee (CUIMC), Wenhuo Hu (Memorial Sloan Kettering Cancer Center, New York, NY), Justin T. Matulay (CUIMC), Mark V. Silva (CUIMC), Tomasz B. Owczarek (CUIMC), Kwanghee Kim (MSKCC), Chee Wai Chua (CUIMC), LaMont J. Barlow (CUIMC), Cyriac Kandoth (MSKCC), Alanna B. Williams (CUIMC), Sarah K. Bergren (CUIMC), Eugene J. Pietzak (MSKCC), Christopher B. Anderson (CUIMC), Mitchell C. Benson (CUIMC), Jonathan A. Coleman (MSKCC), Barry S. Taylor (MSKCC), Cory Abate-Shen (CUIMC), Hikmat Al-Ahmadie (MSKCC), and David B. Solit (MSKCC).
This work was supported by funds from the National Institutes of Health (CA199662, CA193442, CA193313, and OD020355), Urology Care Foundation Research Scholar Award, Urology Care Foundation Residency Research Award, Cycle for Survival, Bladder Cancer Advocacy Network, TJ Martell Foundation, and Linda and Ilan Kaufthal and the Russell Berrie Foundation.
Dr. Solit is a consultant for Pfizer and Loxo Oncology. Drs. Shen, Barlow, and Chua are inventors of U.S. patent application 15/288,871, which is related to this work. The authors report no other potential conflicts of interest.
Media Contact:
Office of Communications [email protected]



