Each year in the United States, there are less than 3,500 people who are diagnosed with a primary melanoma inside the eye, and only 350 diagnosed with retinoblastoma — making eye cancers so uncommon that many physicians may never encounter a single case. For Brian P. Marr, M.D., director of Ophthalmic Oncology in the Department of Ophthalmology at NewYork-Presbyterian and Columbia, seeing these patients is a daily occurrence.
“There are probably fewer than 20 physicians in the country who practice ocular oncology full time,” says Dr. Marr, who is a global leader in the field. “These cancers are rare and technically demanding, and most ophthalmologists may only see one or two cases in their careers. There’s a steep learning curve, and patients can’t afford for their doctor to be treating the disease for the first time.”
Dr. Marr joined NewYork-Presbyterian and Columbia in 2017, following clinical and leadership roles at Wills Eye Hospital and Memorial Sloan Kettering Cancer Center, and has since helped build one of the country’s few high-volume ophthalmic oncology centers. Every year, Dr. Marr’s team handles about 3,500 to 4,000 patient visits and 250 to 350 complex surgeries, and they offer a full spectrum of care for a range of conditions, including superficial conjunctival tumors, retinoblastoma, uveal melanoma, and advanced orbital tumors.
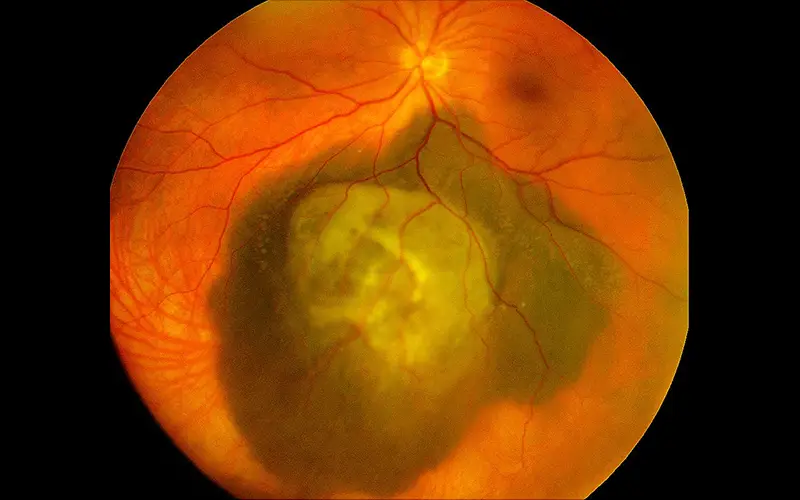
Image showing choroidal melanoma of the left eye. The ophthalmic oncology team at NewYork-Presbyterian and Columbia participates in every major clinical trial currently available for uveal melanomas.
“We treat patients with conditions that can threaten both their vision and their lives,” says Lauren Yeager, M.D., an ophthalmic oncologist at NewYork-Presbyterian and Columbia who works with Dr. Marr to treat both adult and pediatric patients. “We have the foundational pillars to be a center of excellence and the data to support it. What makes this possible is the partnership we’ve built across disciplines.”
Combining Comprehensive Care With Advanced Treatment for Better Patient Outcomes
Dr. Marr’s experience handling complex cases brings in patients from across the globe; about one-third are referred to him from outside the state or country. Patients are treated with a multidisciplinary approach. Every week, the team convenes an ophthalmic oncology-specific tumor board that includes specialists from neurosurgery, otolaryngology, dermatology, interventional radiology, medical and radiation oncology, pediatric oncology, anesthesiology, and pathology to develop coordinated treatment plans.
“It’s common for us to do combined cases with neurosurgery, or dermatology for cases that involve the eyelid,” says Dr. Marr. “We’re also experts in intra-arterial chemotherapy for retinoblastoma, which involves a lot of collaboration with interventional radiologists.”
The ability and experience to execute advanced treatment options is another major contributor to patient success. NewYork-Presbyterian and Columbia is, for instance, among a limited number of centers worldwide offering intra-arterial chemotherapy — a technique Dr. Marr helped refine — in which a catheter is threaded through the femoral artery to deliver high-dose medication directly to the ophthalmic artery, which enables children to avoid enucleation.
Dr. Marr and team also pioneered a chemotherapy plaque implant for retinoblastoma, which is a device positioned externally on the eye that delivers targeted treatment without intracranial vascular access or systemic exposure. NewYork-Presbyterian and Columbia is currently the only U.S. center to offer this treatment, Dr. Marr says.
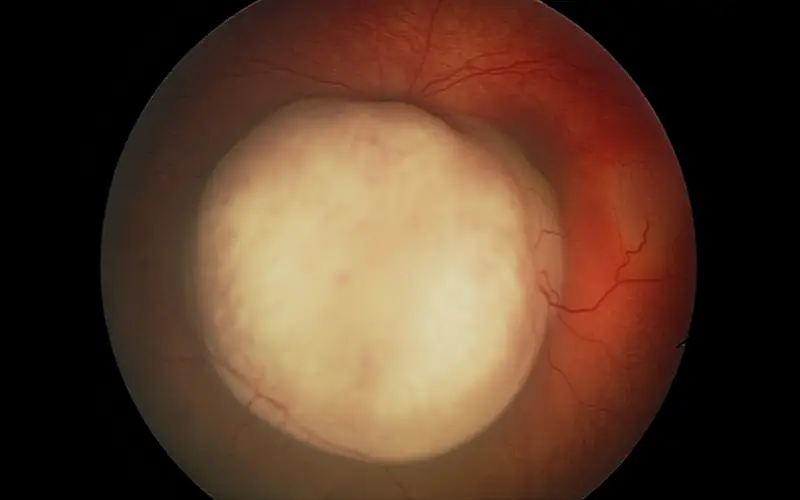
Image showing retinoblastoma of the left eye. Dr. Marr and team have pioneered retinoblastoma treatments such as intra-arterial chemotherapy and a chemotherapy plaque implant.
NewYork-Presbyterian and Columbia’s role as a leading academic medical center also means patients have additional treatment options through access to the latest clinical trials. The ophthalmic oncology division participates in every major clinical trial currently available for uveal melanoma, including studies that test neoadjuvant therapies, immunotherapies, and vision-preserving alternatives to radiation and surgery. Among these are the IDEAYA trial, which evaluates the protein kinase C inhibitor darovasertib as a neoadjuvant therapy before enucleation; and the AURA trial, which tests AU-011, a nanoparticle-laser therapy for small tumors.
“These clinical trials are so imperative to treating these rare, vision-threatening diseases,” says Dr. Yeager. “The AURA trial will help us treat small tumors early to save people’s vision, while IDEAYA will help us shrink tumors so we can treat them locally and save the eye. In the treatment of uveal melanoma, these are a huge step forward.”
Progress in a Rapidly Evolving Field
While current therapies offer incremental survival benefits for metastatic uveal melanoma, the pace of progress is accelerating. Advances in tumor genetics are enabling clinicians to identify high-risk patients earlier and explore more personalized treatment approaches, including targeted adjuvant strategies.
The ophthalmic oncology team is one of the few centers in the U.S. to use gene expression profiling for uveal melanoma, which uses a small sample removed at the time of tumor biopsy to determine a person’s probability of developing metastatic disease. Not only do the results help guide patient follow-up care, but they also help doctors determine if there are therapies in trial that can stop or slow progression of disease.
In January 2022, the FDA also approved tebentafusp as the first systemic therapy for unresectable or metastatic uveal melanoma; NewYork-Presbyterian and Columbia was an enrolling site in the pivotal trial that led to its approval and remains active in research to improve outcomes in both survival and vision preservation.
The culmination of the ophthalmic oncology teams’ leadership in advanced techniques, clinical trial involvement, and forward-thinking innovation is in its patient outcomes: For the past eight years, almost none of the division’s patients have experienced uveal melanoma recurrence.
“We’ve come a long way from where we started,” Dr. Marr says. “I used to meet patients with metastatic disease and knew I wouldn’t see them six months later. Now I’m following some of the same patients for years. It gives me hope for what’s coming next.”





