Nearly 20 years ago, Y. Pierre Gobin, MD, Director of Interventional Neuroradiology at NewYork-Presbyterian/Weill Cornell Medicine, along with other physicians and researchers at Memorial Sloan Kettering Cancer Center, were trying to find innovative approaches to treat hard to reach tumors. They conceived of a new way to use intra-arterial chemotherapy (IAC): as a potential cure for retinoblastoma, a rare eye cancer that accounts for 2% of childhood cancers and, if left untreated, can extend beyond the eye and be deadly. Back then, 95% of children with retinoblastoma — which is most often diagnosed between the ages of 2 and 6 — lost one or both eyes to the disease.
“Intra-arterial chemotherapy is an old idea that comes from the 1960’s,” says Dr. Gobin. “I tried to use this approach in the brain many years ago for glioblastoma, but that disease was too complex to be treated by this approach. However, I knew that this approach had the potential to work for other tumors. In 2006, we started testing intra-arterial chemotherapy in retinoblastoma, and it’s been extremely successful. Now we are testing it for use in other types of cancer.”
Intra-arterial chemotherapy is an old idea that comes from the 1960’s. I tried to use this approach in the brain many years ago for glioblastoma, but that disease was too complex to be treated by this approach. However, I knew that this approach had the potential to work for other tumors.
— Dr. Y. Pierre Gobin
Here’s how Dr. Gobin’s research two decades earlier helped lay a foundation for the current trial of IAC in treating choroid plexus carcinoma, a rare brain cancer that typically forms in infancy.
The Retinoblastoma Success Story
Surgical enucleation, intravenous chemotherapy, and external beam radiation therapy were once mainstays of treatment for retinoblastoma. However, those treatments could lead to loss of the affected eyes. Dr. Gobin and David Abramson, MD, one of his colleagues from Memorial Sloan Kettering Cancer Center, suspected that finding a way to deliver chemotherapy at the exact site of the tumor could help preserve the eye, which led to the investigation into intra-arterial chemotherapy (IAC), also referred to as ophthalmic artery chemosurgery.
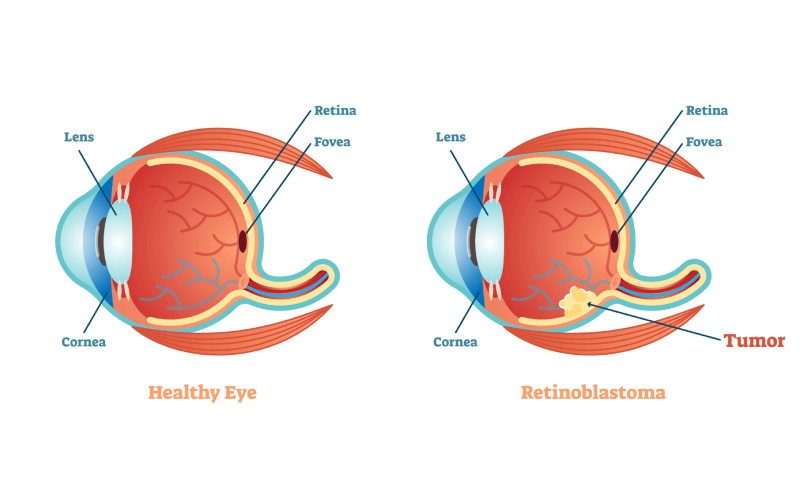
Retinoblastoma occurs when nerve cells in the retina undergo genetic mutations, causing the cells to rapidly grow and multiply and form a tumor.
The technique involves threading a catheter through the femoral artery and advancing it to the ophthalmic artery. Chemotherapy drugs are then injected through the catheter directly into the tumor, sparing the child from the systemic toxicity of intravenous chemotherapy and side effects of external beam radiation therapy.
“We knew that retinoblastoma is a chemo-sensitive tumor fed by only a few blood vessels, so it was easy to place the catheter in those vessels to inject the chemotherapy,” says Dr. Gobin.
Below is a brief timeline of the IAC research from Dr. Gobin and his fellow researchers:
- 2008: The first results demonstrating the feasibility and efficacy of IAC with melphalan.
- 2011: Combining melphalan and topotecan result in an 81.7% event-free survival rate, versus just 58.4% for eyes with treatment failure following IV chemotherapy and radiation. They also began publishing findings on the use of the current three-drug regimen — carboplatin, melphalan, and topotecan — showing that no patients developed metastasis 14 months after treatment and confirming the 88% eye survival rate.
- 2012: Investigators report that the approach is also useful in children with retinal detachment, promoting retinal reattachment and preventing enucleation in 88% of patients.
- 2018: By this time, Dr. Gobin and his fellow researchers report that 75% of eyes treated with IAC remain free of recurrence and more than 90% can be saved. When recurrence does occur, it typically happens in the first year after treatment. They can now treat vitreous seeding of retinoblastoma with intra-vitreal injections of chemotherapy to enhance outcomes.
Today, intra-arterial chemotherapy for retinoblastoma is performed in more than 70 countries around the world. It can be done as an outpatient procedure, spares patients from the immune dysfunction and growth and development delays associated with systemic chemotherapy, and not only saves eyes, but maintains vision. Physicians are now able to save affected eyes in 95% of patients, while only 5% of children require enucleation.
Intra-Arterial Chemotherapy (IAC) Shows Potential for Choroid Plexus Carcinoma
Dr. Gobin’s research with retinoblastoma became a building block for the promise of IAC as an effective therapy for other brain tumors with similar vasculature. Based on the success of IAC for retinoblastoma, Mark Souweidane, MD, Director of Pediatric Neurological Surgery at NewYork-Presbyterian/Weill Cornell Medicine, began working with Dr. Gobin to use IAC as a treatment for choroid plexus carcinoma, a rare brain cancer in which tumors form on the ventricles of the brain, typically in infancy.
“Dr. Gobin’s work in retinoblastoma served as the building block for this trial,” says Dr. Souweidane. “The success of that work gave me hope that we could adopt this very effective therapy and target it toward a different malignancy.”
Dr. Gobin’s work in retinoblastoma served as the building block for this trial. The success of that work gave me hope that we could adopt this very effective therapy and target it toward a different malignancy.
— Dr. Mark Souweidane
The choroid plexus is responsible for producing cerebrospinal fluid. Choroid plexus carcinoma (CPC) and atypical choroid plexus papilloma (ACPP) are extremely rare, accounting for just 3% of pediatric brain tumors. Unlike other brain tumors, cancer in this area arises from a cell of origin in the brain. Children with CPC or ACPP often present with hydrocephalus and extremely large voluminous tumors during their first year of life. Once diagnosed, they require urgent treatment to address the large mass’ effect in the brain.
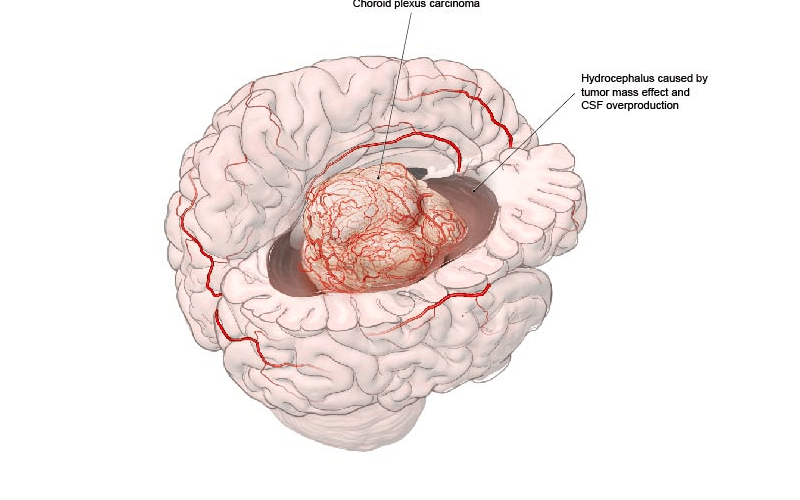
A choroid plexus carcinoma presenting with symptoms of hydrocephalus.
Traditional treatment has been total surgical resection, which achieves the best chance of a successful outcome, but is fraught with complications. "Low circulating blood volume combined with a large voluminous hypervascular tumor is a difficult combination," explains Dr. Souweidane. "As many as 10% of kids lose their life from blood loss during surgery. Another 40% to 50% leave the operating room with a subtotal resection and are destined to fail adjuvant therapy." The three-year event-free survival for newly diagnosed choroid plexus carcinoma is just 30% to 40%.
Knowing choroid plexus carcinoma tumors have a similar chemosensitivity and blood supply as retinoblastoma tumors, Dr. Souweidane began working with Dr. Gobin and Jared Knopman, MD, Director of Cerebrovascular Surgery and Interventional Neuroradiology at NewYork-Presbyterian/Weill Cornell Medicine, to explore how an intra-arterial approach could be applied to these tumors.
"Like retinoblastoma, choroid plexus carcinoma are supplied by only a few blood vessels,” says Dr. Gobin. “We're applying our experience in this area to evaluate intra-arterial chemotherapy and see if we can help these patients, too."
In 2023, the world’s first clinical trial assessing IAC for ACPP and CPC prior to surgery was launched at NewYork-Presbyterian/Weill Cornell Medicine, with Dr. Souweidane serving as the principal investigator. In this phase I trial, concentrated doses of IAC are being delivered directly into the choroid plexus tumors to shrink them, reduce blood flow, and make surgical resection safer and more effective for young patients.
Essential to the procedure is the use of a microcatheter about the diameter of angel hair pasta that is threaded through the femoral artery to the choroidal arteries. Once in place, the same drug trio used to treat retinoblastoma — carboplatin, melphalan, and topotecan — is infused for 20 to 30 minutes per blood vessel. The entire IAC procedure takes up to two hours.
Several weeks after this occurs, an angiogram is performed to assess the size of the tumor and the blood supply. If necessary, Dr. Knopman will go in and use a liquid embolic agent to shut down the blood supply to make surgery even safer for the patients. Then, as the final step in the trial, Dr. Souweidane will perform a gross total excision of the tumor.
Results from the first patient enrolled in the trial, a young boy diagnosed with CPC, shows promise. After receiving IAC, his angiogram showed a significant reduction in tumor blush and size over five weeks. His tumor was removed without incident, and today he is living with no evidence of disease. "He has a long road ahead of him, but from where he was when he came here to how he's doing now, it is gratifying to see him doing so well," says Dr. Souweidane.
He adds that clinical innovations pursued by the NewYork-Presbyterian/Weill Cornell Medicine’ interventional radiology program have contributed to the success of IAC. "We have the most talented interventionalists one could ever ask for,” he says. “They have advanced the use of miniature devices that makes these procedures feasible even in our youngest, tiniest patients.”
Recruitment, however, has been difficult because the cancer is so rare, but the trial is still enrolling patients. "We are now trying to reach physicians, parents, and the worldwide community of people looking for better outcomes for patients with malignant choroid plexus tumors,” he says.
We have the largest worldwide experience in intra-arterial chemotherapy, and it all started with Dr. Gobin and his colleagues two decades ago. We're building on our long track record of making treatments less invasive, targeted, and safer, which is what interventional neuroradiology is all about.
— Dr. Jared Knopman
The evolution and adoption of IAC exemplifies how innovation in the treatment of one disease can be used to benefit so many others. "We have the largest worldwide experience in intra-arterial chemotherapy, and it all started with Dr. Gobin and his colleagues two decades ago," asserts Dr. Knopman. "We're building on our long track record of making treatments less invasive, targeted, and safer, which is what interventional neuroradiology is all about."
If you have a patient with a choroid plexus tumor and you would like more information about referring them to the phase 1 clinical trial, contact Colleen Sanders.






