Mitral valve disease affects more than 24 million people around the world and has an especially high burden of morbidity and mortality in developing countries because of the prevalence of rheumatic heart disease caused by untreated streptococcal infections. These patients often require surgical mitral valve replacement at a young age, but current valve options aren’t optimal for patients under 65.
A recent clinical trial, however, demonstrates the efficacy of a novel mitral valve made of synthetic polymer leaflet materials that could provide a better option for younger patients. One-year results were shared as a late-breaking clinical trial presentation at the Cardiovascular Foundation’s New York Valves 2025 Structural Heart Summit.
“There’s a huge rationale for polymer valves,” says Isaac George, M.D., cardiothoracic surgeon and co-director of the Structural Heart and Valve Center at NewYork-Presbyterian and Columbia, who is the global principal investigator of the trial. “There’s a population of patients in developing countries and in the Western world who have a need for a valve that will last longer. We ultimately want tissue-valve quality of life with the kind of hemodynamics and long-term durability of a mechanical valve.”
The Challenges of Current Valve Replacements
Mechanical valves and bioprosthetic valves, the two most common options, both pose specific challenges for younger patients. Mechanical valves are the most durable, but they require lifelong anticoagulation therapy, which poses bleeding risks for women of reproductive age; additionally, access to the required medications can be difficult in developing countries. Bioprosthetic valves made from animal tissue, meanwhile, require little or no medication but degenerate over time, which means reoperation every five to 10 years.
These valves are not subject to the immunologic issues that we have with animal tissue, and they potentially function more like human tissue than the inflexible leaflets found in mechanical valves.
— Dr. Isaac George
The novel synthetic polymer leaflet valves hold promise to resolve these challenges. They are made of a siloxane polyurethane compound that more closely mimics human valves, are biocompatible, and resist calcification. “These valves are not subject to the immunologic issues that we have with animal tissue, and they potentially function more like human tissue than the inflexible leaflets found in mechanical valves,” says Dr. George.
The way the valve is manufactured can also help resolve global access issues. Its design incorporates a polyetheretherketone (PEEK) radiopaque frame with a PTFE sewing ring, which is dip-cast into the polymer to form the leaflets. A single-unit robot can produce a valve in about 24 hours, making it more scalable than tissue-based valves that require hand sewing. “Tissue valves are very labor intensive and expensive. By reducing the human component, you reduce errors and increase efficiency,” says Dr. George. “And the robot is not bound by geography, so you could potentially place them in locations globally, as long as there is electricity.”
One-Year Clinical Trial Results
In the prospective, single-arm trial, surgeons across eight clinical sites in India performed mitral valve replacement surgery using the novel polymer valve for 67 patients who had moderate to severe mitral valve stenosis, regurgitation, or mixed pathology; 64% of patients were female, and 48% of them were of childbearing age. The findings were recently published in the Journal of the American College of Cardiology.
All patients in the study underwent complete sternotomy to replace their current valve with the new polymer valve and were put on anticoagulation therapy to prevent thrombosis, although the plan is to reduce anticoagulation gradually over time, says Dr. George. At one year, the valve demonstrated stable hemodynamics. Echocardiography showed that both the effective orifice area and the mean inflow gradient had statistically significant improvement at 30 days after surgery, which was maintained at one year. Mitral regurgitation was reduced to mild or less in 98% of patients in the study.
Symptoms and functioning also improved for nearly all patients in the study:
- Before valve replacement, all patients were classified as New York Heart Association (NYHA) functional class II or worse, indicating at least some limitations in ordinary physical activity.
- By six months, about 70% had improved and were classified as NYHA functional class I, meaning they had no limitations on activity. By one year, that number had grown to more than 80%.
- Six-minute walk test distances improved from 298.1 meters on average at baseline to 494.8 meters at one year.
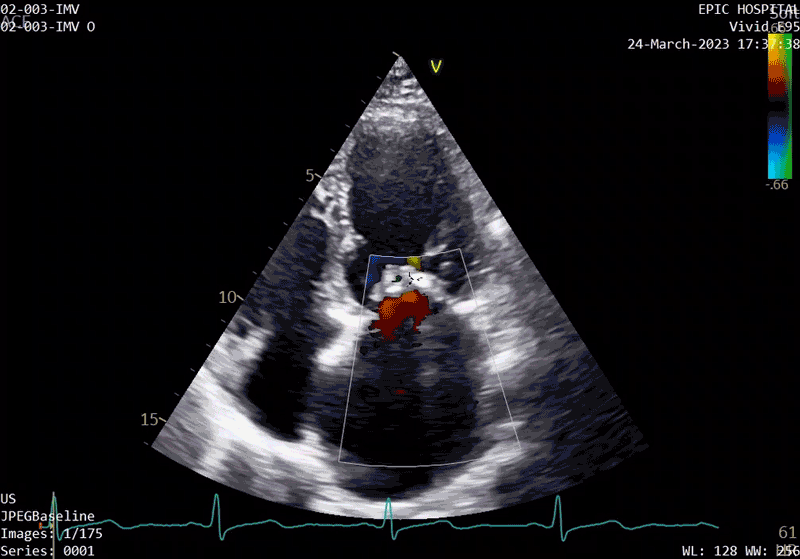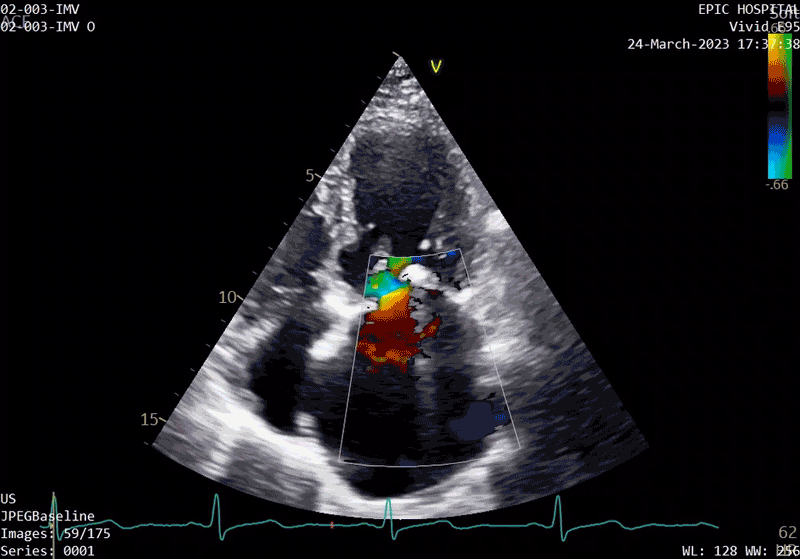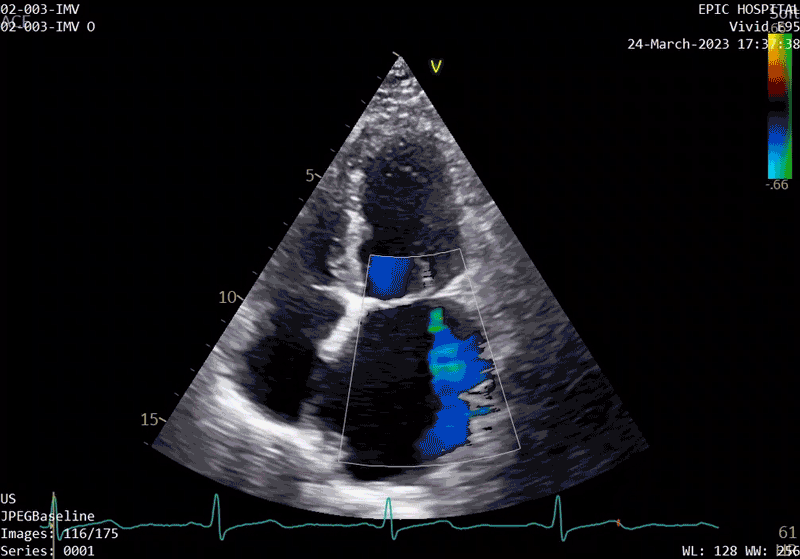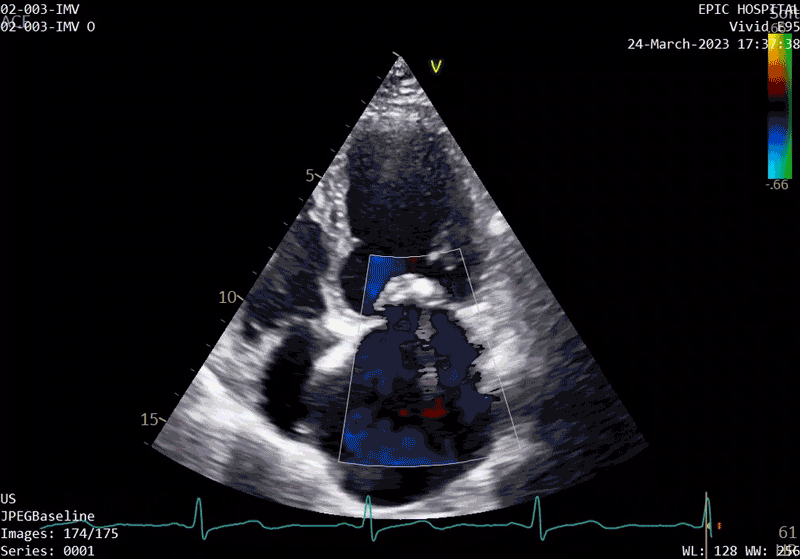
A sample patient echocardiogram at baseline before surgery shows a classic rheumatic mitral valve with stenosis.
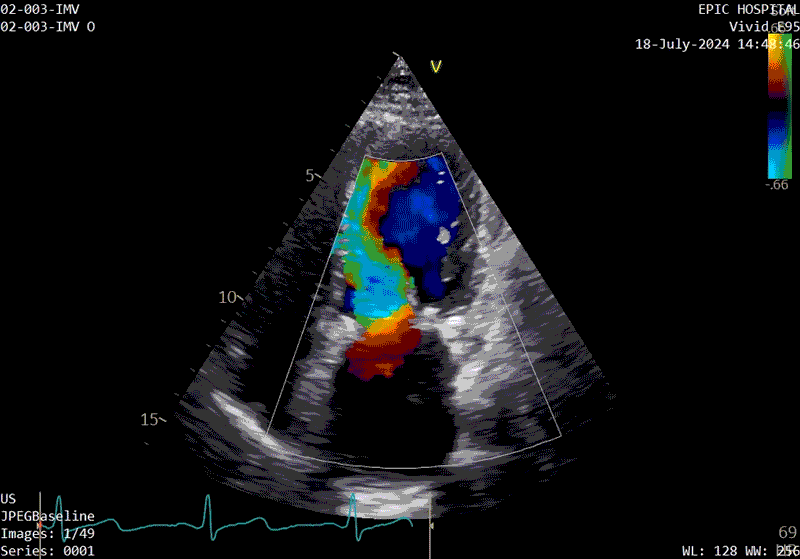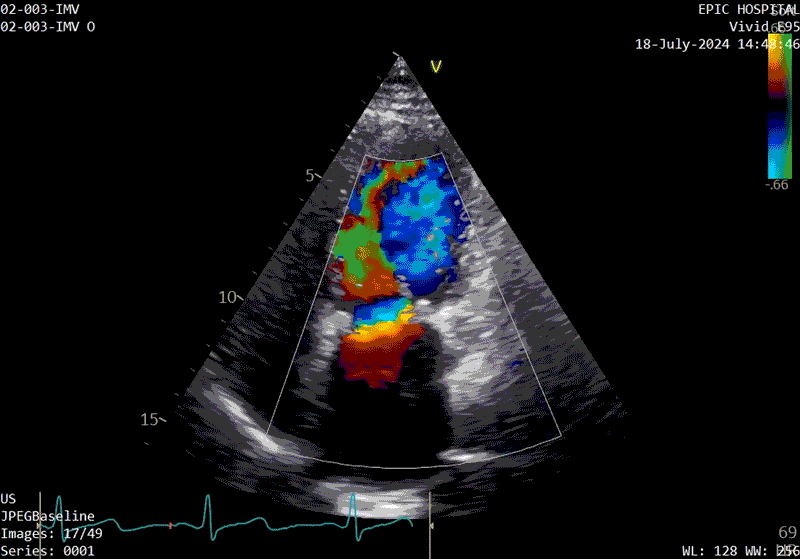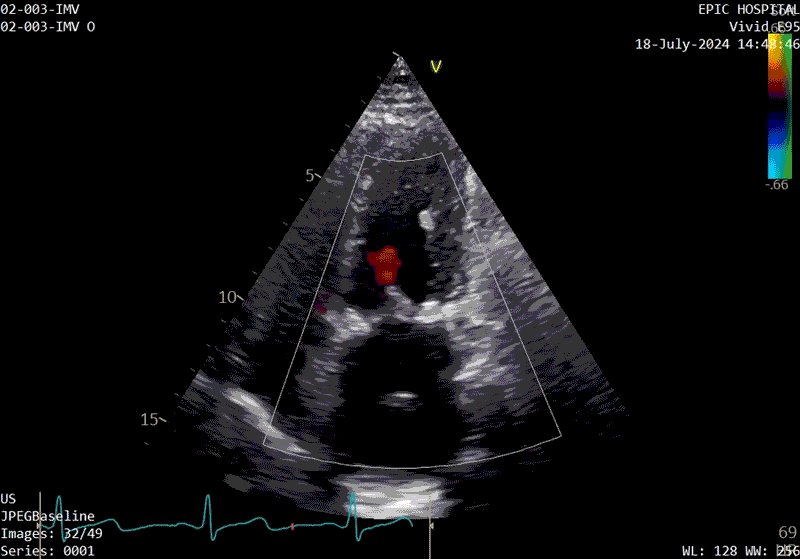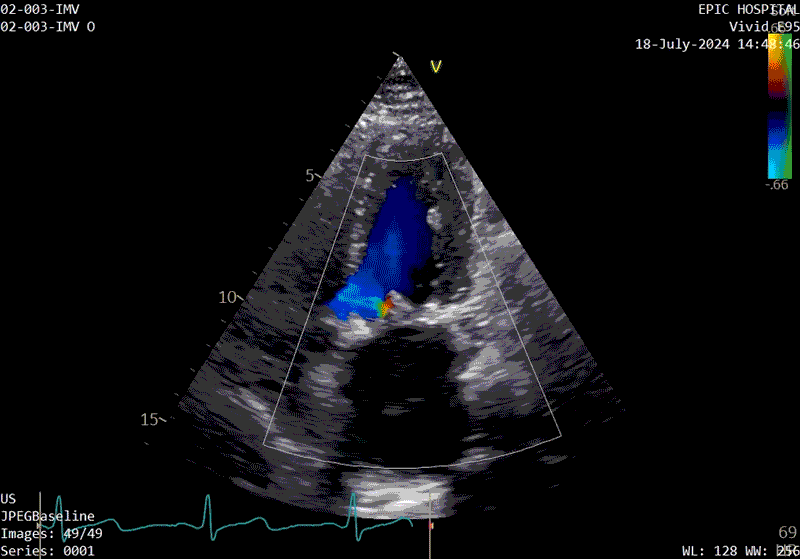
The patient’s echo at one-year after polymer valve implantation. After replacement with a polymer valve, rapid ejection and laminar flow pattern into the left ventricle can be seen, and the opening and closing of the leaflets in the cardiac cycle are relatively quick compared with traditional valves.
Safety was comparable to commercial bioprosthetic surgical valves. At the one-year mark, all-cause mortality was 9.1% and there were no valve-related deaths. There were also no valve reoperations, no structural valve deterioration, and no cases of endocarditis. There were five thromboembolic events, including three ischemic strokes and two patients who experienced valve thrombosis.
The data has already led to commercial approval of the polymer valve in India, and it is expected to be rolled out to other developing countries in South Asia and the Middle East, says Dr. George.
Future Trials and Applications for the Polymer Valve
The promising results have laid the foundation for a larger study in the U.S. to confirm the safety and clinical outcomes seen in India. The U.S. study, which will also be led by Dr. George, will be a single-arm investigational device exemption (IDE) study aimed at achieving approval from the U.S. Food and Drug Administration.
“Where the valve has the most value right now is in developing countries, where the huge burden of rheumatic heart disease leaves many young patients in need of life-changing surgery,” says Dr. George. However, the technology could expand to other types of polymer heart valves in the future. “We can see a scenario where this kind of leaflet technology gets used perhaps for an aortic, pulmonic, or tricuspid valve for a pediatric patient with a young, active immune system who needs durability.” The design may also allow for future transcatheter implantation, he adds. The company that developed the polymer valve is also developing a transcatheter aortic valve replacement (TAVR) device.
“This is just the start of our investigation of this technology, to prove that this polymer can translate into long-term results,” says Dr. George. “Ultimately, we want to push the field forward to make things better for patients with valve disease. We don’t want to settle for the status quo; we want to innovate and keep pushing limits.”




