An estimated 25 percent of maternal deaths worldwide – and approximately 11 percent in the United States – result from postpartum hemorrhage, making it the leading cause of maternal mortality. While a number of factors may contribute to abnormal postpartum uterine bleeding, uterine atony is the most prevalent, accounting for 80 percent of postpartum hemorrhages and occurring in about one in 40 births in the United States. A novel device is now available to address this major complication quickly and safely. The Jada® System is a vacuum-induced hemorrhage control device that provides a rapid and effective treatment option for abnormal postpartum uterine bleeding and postpartum hemorrhage. In October 2021, the Food and Drug Administration (FDA) granted Special 510(k) clearance for the latest version of the Jada System based on the results of the pivotal PEARLE IDE Study led by principal investigators Mary D’Alton, MD, and Dena Goffman, MD.
Dr. D’Alton is Chair of the Department of Obstetrics and Gynecology, Columbia University Irving Medical Center, and Director of Services, Sloane Hospital for Women at NewYork-Presbyterian. Dr. Goffman is Chief of Obstetrics, Sloane Hospital for Women, and Vice Chair for Quality and Patient Safety in the Department of Obstetrics and Gynecology, NewYork-Presbyterian/Columbia University Irving Medical Center.

Dr. Mary D’Alton

Dr. Dena Goffman
“Given the results of the PEARLE Study, the Jada System offers an important new tool as we continue to strive to decrease rates of severe maternal morbidity and mortality from hemorrhage in the United States,” says Dr. D’Alton, who is one of the country’s foremost experts on maternal fetal medicine and a leading advocate for efforts to reduce the rate of maternal mortality and morbidity. Dr. D’Alton also serves as Co-Chair of the Safe Motherhood Initiative, which has implemented changes in New York State hospitals to reduce maternal deaths and serious complications.
Dr. Goffman, who has a longstanding interest in obstetric hemorrhage, served as Co-chair of the Obstetric Hemorrhage Work Group for the New York State Safe Motherhood Initiative and was an active member of the OB Hemorrhage Clinical Advisory Work Group for the NYS Department of Health. She also was a core member of the original National Partnership for Maternal Safety Obstetric Hemorrhage Work Group, co-authored the Postpartum Hemorrhage ACOG Practice Bulletin, and is currently working with a multidisciplinary team on revisions to the Alliance for Innovation on Maternal Health (AIM) Obstetric Hemorrhage Bundle.
“Our study found that using low-level suction to promote uterine contraction, rather than the compression used by other available devices, helped to resolve postpartum hemorrhage safely and rapidly,” says Dr. Goffman. “These findings suggest that the new device treats hemorrhage quickly and effectively, allowing mothers to resume normal activities shortly after delivery. Having a new option to treat women with severe postpartum bleeding could help reduce morbidity and mortality associated with this all-too-common complication of childbirth.”
Jada System: How the Device Works
The Jada System is an intrauterine device that uses low-level vacuum to encourage the natural forces of uterine contraction to control abnormal postpartum uterine bleeding or postpartum hemorrhage after childbirth. Made of soft silicone, the 41 cm long device consists of an elliptical intrauterine loop on the distal end of a tube. A soft shield covers the outside of the loop to protect uterine tissue and to prevent occlusion of the pores located on the inner surface of the loop. The pores enable the vacuum to collapse the uterus and evacuate any pooled blood. A cervical seal, located between the intrauterine loop and the tube, limits the vacuum to only the uterine cavity. The proximal end of the tube connects to sterile vacuum tubing that empties into an inline canister.
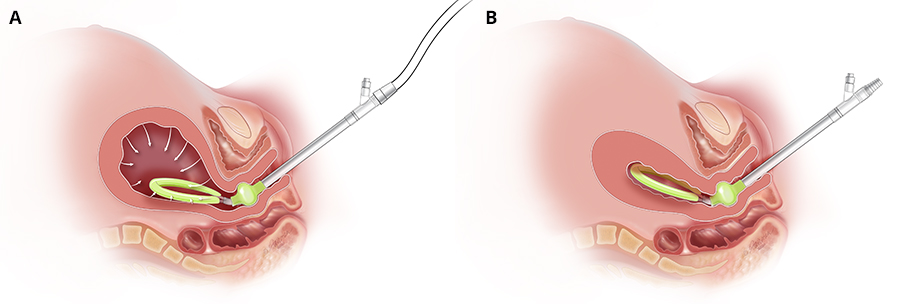
(A) Jada with vacuum being applied (B) Jada with uterus collapse (Courtesy of Organon)
After a vaginal birth, a manual sweep of the uterine cavity is performed to remove any clots. The device is then placed transvaginally and the cervical seal is filled with sterile fluid to ensure a seal for vacuum. The evacuated blood is measured as it passes through the tubing into the canister. After at least 60 minutes of vacuum use, the intrauterine device remains in place for an additional 30 minutes to allow close observation for any return of atony or abnormal bleeding necessitating further treatment before removal. Total therapy duration is determined by the provider based on the patient’s status, including vital signs, uterine tone, and cumulative blood loss.
The PEARLE Study
This multicenter, prospective, single-arm treatment study evaluated the safety and effectiveness of the Jada System. Twelve U.S. hospitals with prestigious maternal fetal medicine and obstetric research programs participated in the study led by Dr. D’Alton and her Columbia colleagues in the Department of Obstetrics and Gynecology. The 99 unique investigators treated 106 patients and performed a six-week follow-up visit. The primary effectiveness endpoint of the study was met in 94 percent of cases.
Additionally, abnormal bleeding was controlled in a median of three minutes after connection to vacuum. Total treatment time, including the time to control bleeding plus device in-dwelling time after bleeding control, was 3.2 hours on average, with minimal evacuation of blood from the uterus after initiation of treatment.
The primary safety endpoint of the study was also met, with a low rate of adverse events, all of which were resolved without serious complications.
The investigators reported that the device is easy to use (98 percent) and would recommend it (97 percent). The study findings were published in the November 2020 issue of Obstetrics and Gynecology.
“The Jada System is a solution that can be deployed quickly and easily,” adds Dr. D’Alton. “I’d like to thank my fellow investigators for their contributions to the study and for their unwavering commitment to improving outcomes for mothers. We also thank the many expectant mothers who consented to be enrolled in the study, as without their willingness to participate, we could not have completed this important clinical evaluation.”



