The device mentioned in this article received approval by the U.S. Food and Drug Administration in November 2023.
According to a new study led by faculty at NewYork-Presbyterian/Columbia and the Université de Paris, the use of ultrasound renal denervation resulted in a consistent reduction of daytime ambulatory blood pressure by an average of 8.5 points among middle-aged individuals with hypertension.
While physicians generally prescribe lifestyle changes and medications to lower blood pressure, about one-third of hypertensive patients are unable to control their blood pressure despite these interventions. “Even with the number of drugs available and lifestyle modifications, there are still so many patients whose hypertension remains uncontrolled based upon national and worldwide statistics,” says Ajay J. Kirtane, MD, SM, an interventional cardiologist and Director of Cardiac Catheterization Laboratories at NewYork-Presbyterian/

Ajay J. Kirtane, MD, SM
Hypertension in middle age is thought to be caused in part by overactive nerves in the kidneys, which trigger water and sodium retention and release hormones that can raise blood pressure. Current antihypertensive drugs, however, do not target the renal nerves directly. Ultrasound-based renal denervation therapy, which is delivered endovascularly via a catheter-based procedure, is used to ablate the nerves of the kidneys to decrease sympathetic nerve activity, thereby disrupting signals that lead to hypertension.
“Renal ultrasound could be offered to patients who are unable to get their blood pressure under control with lifestyle changes and drug therapy to potentially help prevent events such as heart failure, stroke, heart attack, and irreversible kidney damage that can result,” says Dr. Kirtane. “This approach could represent a major change in our clinical practice, but it needs to be implemented as an adjunct to existing treatments that we know already work. For instance, if a patient came to see me for the first time with elevated blood pressure, I would try the usual treatment protocols first. If those didn’t work despite best efforts, then potentially the patient could be considered for ultrasound renal denervation.”
According to Dr. Kirtane, establishing the scale and consistency of ultrasound renal denervation across the spectrum of patients with uncontrolled hypertension is clinically important. Over the past several years, Dr. Kirtane has served as a Principal or Co-Principal Investigator evaluating the ReCor Medical Paradise® System in three sham-controlled trials: RADIANCE-HTN SOLO, RADIANCE-HTN TRIO, and RADIANCE II. The RADIANCE-HTN TRIO studied patients with resistant hypertension, while RADIANCE-HTN SOLO and RADIANCE II studied patients with mild-moderate hypertension. The data from these three trials demonstrated an overall reduction in daytime ambulatory systolic blood pressure in the ultrasound renal denervation group.
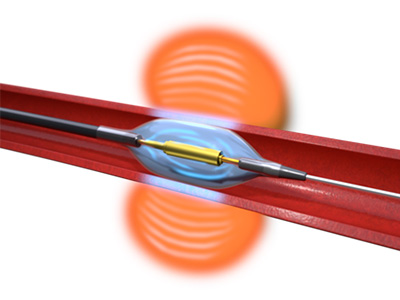
The Paradise Renal Denervation System administers ultrasound energy to the tissue surrounding the artery to decrease the signaling of over-active nerves leading to the kidney. The Hydrocooling System provides active cooling from circulating water to protect the artery (image courtesy of ReCor Medical).
“Within the trial, by and large, patients had to be on stable drugs or stable medical regimens for some period of time,” notes Dr. Kirtane. “The reason behind this is to ensure that the patient’s medical regimen remained unchanged as medicines can lower blood pressure and that might obscure the observed effect of the denervation therapy.”
The current study pooled individual patient data from the three trials, which involved more than 500 middle-aged patients with varying degrees of hypertension. Twice as many patients who received the ultrasound therapy reached their target daytime blood pressure (less than 135/85 mmHg) compared with patients in the sham groups.
Having three consistent sham-controlled randomized clinical trials demonstrating that this system can safely lower blood pressure across a range of patients is a very high bar to have met.
— Dr. Ajay Kirtane
The analysis, which was led by Dr. Kirtane and Michel Azizi, MD, PhD, Professor of Vascular Medicine at the Université de Paris, in collaboration with RADIANCE investigators, were published in the February 28, 2023, issue of JAMA Cardiology.
“The results of the RADIANCE clinical trials are meaningful in that they solidify the role of the Paradise ultrasound renal denervation system as an adjunctive therapy for hypertension treatment,” says Dr. Kirtane. “Having three consistent sham-controlled clinical trials demonstrating that this system can safely lower blood pressure across a range of patients is a very high bar to have met. The result was almost identical across the different study groups, which definitively shows that the device can lower blood pressure in a broad range of patients. Not only does the procedure reduce hypertension, it also works synergistically with medication and provides an alternative for patients who cannot tolerate medicines or don't wish to take them. Ultimately, ultrasound renal denervation will provide clinicians another tool to use for the appropriate patient.”
The ultrasound renal denervation system will undergo evaluation by the FDA in the coming months, and Dr. Kirtane anticipates its approval within the next year.




