Paroxysmal supraventricular tachycardia (PSVT), which affects approximately two million Americans, is characterized by sporadic episodes of tachyarrhythmia. Certain intravenous medications, including adenosine, beta blockers, and calcium channel blockers, have long been used for the rapid termination of PSVT. However, these medications must be administered under medical supervision and intravenous access, usually in an emergency department or other acute care setting.
Dr. James Ip
“Currently, no medications are approved for acute termination of paroxysmal supraventricular tachycardia without direct medical supervision,” says James E. Ip, MD, Associate Professor at Weill Cornell Medicine and Director of Cardiac Pacing and Implantable Devices in the Division of Cardiology at NewYork-Presbyterian/
Etripamil is a new investigational drug that may well transform care for patients with PSVT. A rapid acting, L-type calcium channel blocker, etripamil is administered as a nasal spray. As with other non-dihydropyridine calcium channel blockers, etripamil slows atrioventricular node conduction and increases its refractory period. “Because of its rapid onset of action as well as short half-life, it is an ideal medication for patients to self-treat their tachyarrythmias,” says Dr. Ip. “The drug acts very quickly. It is absorbed through the nasal mucosa and achieves peak levels within eight minutes, unlike a pill, which takes a much longer time to have an effect. Etripamil also has a quick offset because the drug is rapidly metabolized by blood esterases.”
Dr. Ip has served as a primary investigator in a series of studies on etripamil, beginning with the NODE-301 trial. “This trial showed that patients were able to safely use etripamil without direct medical supervision and terminate their PSVT most of the time,” says Dr. Ip. “While the trial did not meet the primary five-hour efficacy endpoint, etripamil was shown to have a significant treatment effect in terminating PSVT at earlier timepoints – 54 percent conversion at 30 minutes, consistent with the drug’s pharmacology, and the medication was safe and well tolerated with no serious adverse events reported.”
These results supported the continued clinical development of the etripamil nasal spray, leading to the NODE-302 trial. As reported by Dr. Ip at the 2022 Heart Rhythm Society meeting, this single arm, open label extension study included 105 patients who completed their randomized dose in NODE-301 and were able to use etripamil to treat up to 11 episodes of PSVT without direct medical supervision. The trial showed an adjudicated PSVT conversion rate of 60.2 percent at 30 minutes and 75.1 percent at 60 minutes. There were no cases of syncope, pauses, or atrioventricular block demonstrating continued safety with etripamil without direct medical supervision.
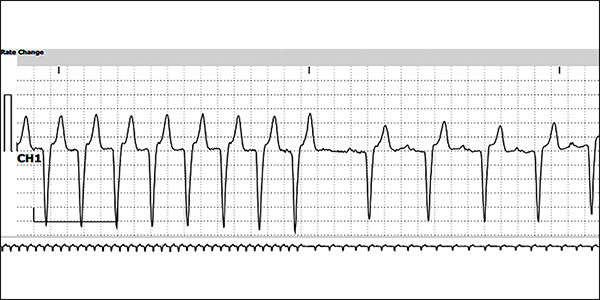
ECG showing etripamil stopping an episode of PSVT (Courtesy of Dr. James Ip)
RAPID Study: Evaluating a Two-Dose Protocol
“With the positive results of NODE-302, we then decided to see if a second dose of etripamil could improve conversion rates without compromising safety,” says Dr. Ip. “When the first dose of the etripamil spray did not work, we gave the patient an opportunity to self-administer a second dose 10 minutes later. The RAPID study was a double dose trial and also placebo controlled.”
In November 2022, Dr. James Ip presented results of the RAPID study at a late-breaking clinical trials session during the American Heart Association Annual Meeting.
“Data from the RAPID trial demonstrated that patients on the etripamil regimen had both a greater degree of conversion and a much faster conversion than those taking placebo,” continues Dr. Ip. “When etripamil was taken in an optional repeat dose combination, the termination rates improved significantly and there were no problems with safety. These findings further support that etripamil could offer patients a meaningful intervention for their PSVT outside of the more costly and inconvenient acute medical care setting.”
The RAPID study enrolled 706 patients across clinical sites in North America and Europe. The safety and tolerability data from the RAPID trial continue to support the potential self-administration of etripamil by patients at home, with findings consistent with those seen in the prior trials. Among the results, presented by Dr. Ip at a late-breaking clinical trials session during the American Heart Association’s 2022 Annual Meeting, were:
- The RAPID trial met its primary endpoint, with 64.3 percent of patients receiving etripamil converting to sinus rhythm within 30 minutes compared to 31.2 percent on placebo
- Median time to conversion was 17.2 minutes for patients treated with etripamil – three times faster than placebo at 53.5 minutes
- Pre-specified analyses of pooled data with the NODE-301 study showed significant reductions in medical interventions and visits to the emergency department for patients who took etripamil
- Safety and tolerability data were consistent with previous etripamil trials, supporting potential self-administration of the optional repeat dose regimen
- The most common randomized treatment emergent adverse events occurring within 24 hours of the drug administration were related to the nasal administration site (i.e., nasal discomfort/congestion)
- Overall, the majority of adverse events were reported as mild (68 percent) to moderate (31 percent), with no reported serious adverse events related to etripamil (i.e., AV block, pauses, or syncope)
“These results demonstrate a potential management strategy for patients to self-treat episodes of paroxysmal supraventricular tachycardia with etripamil in a medically unsupervised setting,” says Dr. Ip. “Ongoing analysis of RAPID open-label period and the upcoming NODE-303 trial will provide more insights into the safety and efficacy of etripamil for recurrent episodes.”