While endovascular renal denervation has been shown to reduce mild-to-moderate hypertension, its value for patients with true resistant hypertension remains unknown. Preliminary results from an international clinical trial of the procedure show promise as a potential therapeutic option for these patients.
Ajay J. Kirtane, MD, a Professor of Medicine at Columbia University Irving Medical Center and Director of the Cardiac Catheterization Laboratories at NewYork-Presbyterian/Columbia University Irving Medical Center, serves as Co-Principal Investigator of the RADIANCE-HTN TRIO trial, a randomized, sham-controlled trial being conducted at 28 tertiary centers in the United States and 25 in Europe.
Dr. Ajay J. Kirtane
“There are a variety of effective medications for lowering blood pressure, but many patients need to take several drugs to control their hypertension,” says Dr. Kirtane. “These drugs can have side effects and adherence can be difficult to maintain. It’s clear that we need additional therapeutic approaches to help uncontrolled patients get their blood pressure under control.”
The study included patients aged 18-75 years with office blood pressure of at least 140/90 mm Hg despite three or more antihypertensive medications, including a diuretic. Eligible patients were switched to a daily, single-pill combination of a calcium channel blocker, an angiotensin receptor blocker, and a thiazide diuretic. After four weeks of this therapy, patients whose daytime ambulatory blood pressure remained higher than 135/85 mm Hg were randomly assigned to either undergo ultrasound renal denervation or a sham procedure.
“Eighty percent of patients continued to take their medication as directed, and while that’s a good adherence rate, it still means that one in five patients wasn’t adherent to the simplified medication regimen,” notes Dr. Kirtane. “Of the 136 patients whose blood pressure remained high after four weeks on the new regimen, 69 were treated with renal denervation and 67 had the sham procedure.”
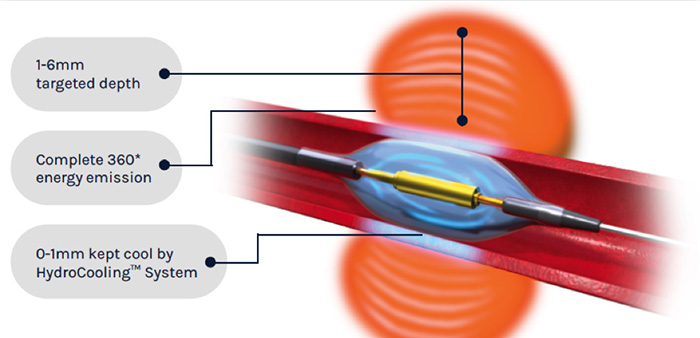
The Paradise Renal Denervation System administers heat from the ultrasound energy to the tissue surrounding the artery to decrease the signalling of over-active nerves leading to the kidney. The Hydrocooling System provides active cooling from circulating water to protect the artery. (Courtesy of ReCor Medical)
Data from the trial was presented at the 2021 American College of Cardiology conference and simultaneously published online in The Lancet. After two months, daytime blood pressure dropped 8 points compared to a 3-point drop in patients who were treated with a sham procedure. Nighttime blood pressure decreased by an average of 8.3 points in the treatment group versus 1.8 points in the sham group.
“For patients with drug-resistant hypertension, a drop in systolic blood pressure of 8 points — if maintained over longer-term follow-up — is almost certainly going to help reduce the risk of heart attack, stroke, and other adverse cardiac events,” says Dr. Kirtane. “These results suggest that renal denervation has the potential to become an important add-on to lifestyle modification and medication therapy, especially for those who have difficulty managing several medications to control their hypertension.”
In a prior parallel study of patients with less severe hypertension, ultrasound renal denervation also reduced blood pressure to a greater extent compared with a sham procedure, with a maintenance of lower blood pressure with less medications needed to achieve this effect.
Ultrasound renal denervation is only available through clinical trials and has not yet been approved for use by the FDA. The ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN) trial will follow patients for five years to determine if the drop in blood pressure is maintained over time.