Targeted Treatment Slows Progression of Rare Connective Tissue Tumor
In a phase three clinical trial, sorafenib stopped progression of desmoid tumors for two years in 80 percent of patients who completed treatment.
Dec 19, 2018
New York
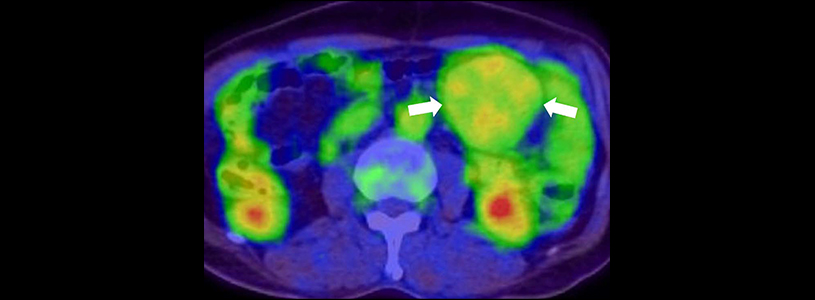
In a phase three clinical trial, a drug called sorafenib stopped progression of desmoid tumors for two years in 80 percent of patients who completed treatment, a significant increase in progression-free survival compared with placebo. (Progression-free survival is the length of time a patient lives without worsening of the disease).
Results of the multicenter trial were published in the New England Journal of Medicine.
There is no standard of care for patients with desmoid tumors. “In general, desmoids are locally aggressive and often painful tumors for which there are no effective therapies,” says Gary K. Schwartz, MD, chief of hematology/oncology at NewYork-Presbyterian/Columbia University Irving Medical Center and a senior author of the paper. “Sorafenib is an oral agent that provides a new means to directly target the ability of desmoid tumors to grow.”
Desmoid tumors (also called aggressive fibromatosis) are abnormal growths that arise in connective tissue and can occur anywhere in the body. Desmoid tumors are not considered cancerous, because they do not spread to other parts of the body. They do not often cause death, but these tumors can cause significant health problems by invading surrounding tissues, causing pain, limiting mobility, and interfering with organ function. About 1,000 new cases, mostly in young adults, are diagnosed in the U.S. each year.
Desmoid tumors are very complex and display a wide range of behaviors, even in the same patient. Some tumors shrink spontaneously, some remain stable, while still others grow aggressively. Treatment varies from patient to patient. In the past, most patients were treated with surgery, but because there is a high risk that the tumor will return, surgery is now mainly used for tumors that are thought to have a low risk of recurrence. Radiation, chemotherapy, and hormonal treatments are sometimes effective in reducing tumor size and alleviating pain.
In this randomized, double-blinded trial, 87 patients with progressive, symptomatic, or recurrent desmoid tumors were given either oral sorafenib or placebo until scans showed disease progression.
Sorafenib is a targeted therapy that acts on tyrosine kinases—enzymes that have been implicated in cancer development—to inhibit the growth of cancer cells and the formation of new blood vessels that support tumor development. The drug has been approved by the FDA to treat certain types of advanced kidney, liver, and thyroid cancer. It has not yet been approved for the treatment of desmoid tumors.
The study is ongoing, but the researchers estimate that progression-free survival after two years is 81 percent in those taking sorafenib, and only 36 percent in those taking placebo. “This is a truly remarkable outcome,” says Schwartz, who is also a professor of oncology at Columbia University Vagelos College of Physicians and Surgeons. “In fact, we have never seen results like this in the treatment of desmoid tumors.”
Side effects, including rash, hypertension, and diarrhea, were more common in patients taking sorafenib than in those taking placebo.
The study was designed to measure sorafenib’s effect on progression-free survival. It did not address whether the drug led to meaningful improvements in pain or other symptoms.
In addition, 22 percent of patients in the treatment group discontinued therapy due to adverse events, such as fatigue and rash.
Additional clinical trials are needed to identify the optimal dosage to reduce the risk of treatment-related adverse events and determine whether an increase in progression-free survival also leads to improvements in pain relief, function, and quality of life.
Researchers are currently analyzing tumor samples obtained in this trial to better understand how sorafenib affects desmoid tumors at the molecular and cellular levels. Understanding sorafenib’s mechanism of action could lead to the development of additional therapies in the future.
About the Paper
The study is titled, “Sorafenib in Advanced and Refractory Desmoid Tumors.”
The other authors are Mrinal M. Gounder (Memorial Sloan Kettering Cancer Center), Michelle R. Mahoney (Mayo Clinic), Brian Andrew Van Tine (Washington University St. Louis), Vinod Ravi (MD Anderson), Steven Attia (Mayo Clinic), Hari Anant Deshpande (Yale), Abha A. Gupta (University Health Network Princess Margaret Cancer Center), Mohammed M. Milhem (University of Iowa), Robert Martin Conry (University of Alabama), Sujana Movva (Fox Chase Cancer Center), Michael J. Pishvaian (MedStar Georgetown University Hospital), Richard Riedel (Duke), Tarek Sabagh (Dayton NCI Community Oncology Research Program), William D. Tap (MSKCC), Natally Horvat (MSKCC), Ethan Basch (University of North Carolina), Lawrence Schwartz (Columbia University), Robert G. Maki (Northwell Cancer Institute), Narasimhan P. Agaram (MSKCC), Robert A. Lefkowitz (MSKCC), Yousef Mazaheri (MSKCC), Rikiya Yamashita (MSKCC) John Joseph Wright (National Cancer Institute), and Amylou C. Dueck (Mayo Clinic).
The study was supported by grants from the National Cancer Institute of the National Institutes of Health (U10CA180821, U10CA180882, and U24CA196171, U10CA180833, U10CA180838, U10CA180857, U10CA180858, UG1CA189850, UG1CA189957, U10CA180820, U10CA180868, U10CA180888, and U10CA180826), Bayer Pharmaceuticals,
Memorial Sloan Kettering Cancer Center, American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and Food and Drug Administration Orphan Products Clinical
Trials Grants Program.
Dr. Schwartz receives consulting fees from Bayer Health Care Pharmaceuticals. Additional disclosures may be found in the paper.
Columbia University Irving Medical Center
Columbia University Irving Medical Center provides international leadership in basic, preclinical, and clinical research; medical and health sciences education; and patient care. The medical center trains future leaders and includes the dedicated work of many physicians, scientists, public health professionals, dentists, and nurses at the Vagelos College of Physicians and Surgeons, the Mailman School of Public Health, the College of Dental Medicine, the School of Nursing, the biomedical departments of the Graduate School of Arts and Sciences, and allied research centers and institutions. Columbia University Irving Medical Center is home to the largest medical research enterprise in New York City and State and one of the largest faculty medical practices in the Northeast. For more information, visit cumc.columbia.edu or columbiadoctors.org.
NewYork-Presbyterian
NewYork-Presbyterian is one of the nation’s most comprehensive, integrated academic healthcare delivery systems, whose organizations are dedicated to providing the highest quality, most compassionate care and service to patients in the New York metropolitan area, nationally, and throughout the globe. In collaboration with two renowned medical schools, Weill Cornell Medicine and Columbia University Vagelos College of Physicians and Surgeons, NewYork-Presbyterian is consistently recognized as a leader in medical education, groundbreaking research and innovative, patient-centered clinical care.
NewYork-Presbyterian has four major divisions:
- NewYork-Presbyterian Hospital is ranked #1 in the New York metropolitan area by U.S. News and World Report and repeatedly named to the Honor Roll of “America’s Best Hospitals.”
- NewYork-Presbyterian Regional Hospital Network comprises hospitals and other facilities in the New York metropolitan region.
- NewYork-Presbyterian Physician Services, which connects medical experts with patients in their communities.
- NewYork-Presbyterian Community and Population Health, encompassing ambulatory care network sites and community healthcare initiatives, including NewYork Quality Care, the Accountable Care Organization jointly established by NewYork-Presbyterian Hospital, Weill Cornell Medicine and Columbia.
For more information, visit www.nyp.org and find us on Facebook, Twitter and YouTube.
Media Contact:
Office of Communications [email protected]


