Tetralogy of Fallot is one of the most common types of congenital heart disease. Its constellation of features — anterior malalignment of the conal septum creating a ventricular septal defect, pulmonary stenosis, right ventricular hypertrophy, and a misplaced aorta — impair blood flow and cause the telltale cyanosis that characterizes the disease. It is treated with surgery, usually between 4 and 12 months of age.
At NewYork-Presbyterian and Weill Cornell Medicine, congenital heart disease specialists consider tetralogy of Fallot to be their "workhorse" for research. That’s because they're using it as a model to evaluate an innovative approach to regenerating cardiomyocytes and cardiac muscle, yielding insights with the potential to improve the care of adults with heart failure. The transformative research is led by Bernhard Kühn, MD, who joined NewYork-Presbyterian and Weill Cornell Medicine in February 2024 as chief of the Division of Cardiology in Pediatrics. He also leads the new Pediatric Institute for Heart Regeneration and Therapeutics, which focuses on pediatric myocardial growth and regeneration.
Addressing an Unmet Need
Even after reparative surgery, children born with tetralogy of Fallot continue to experience poor blood flow through the right ventricle. They face an elevated risk of heart failure and arrhythmia. Research indicates the right ventricle is not growing normally at the cellular level.
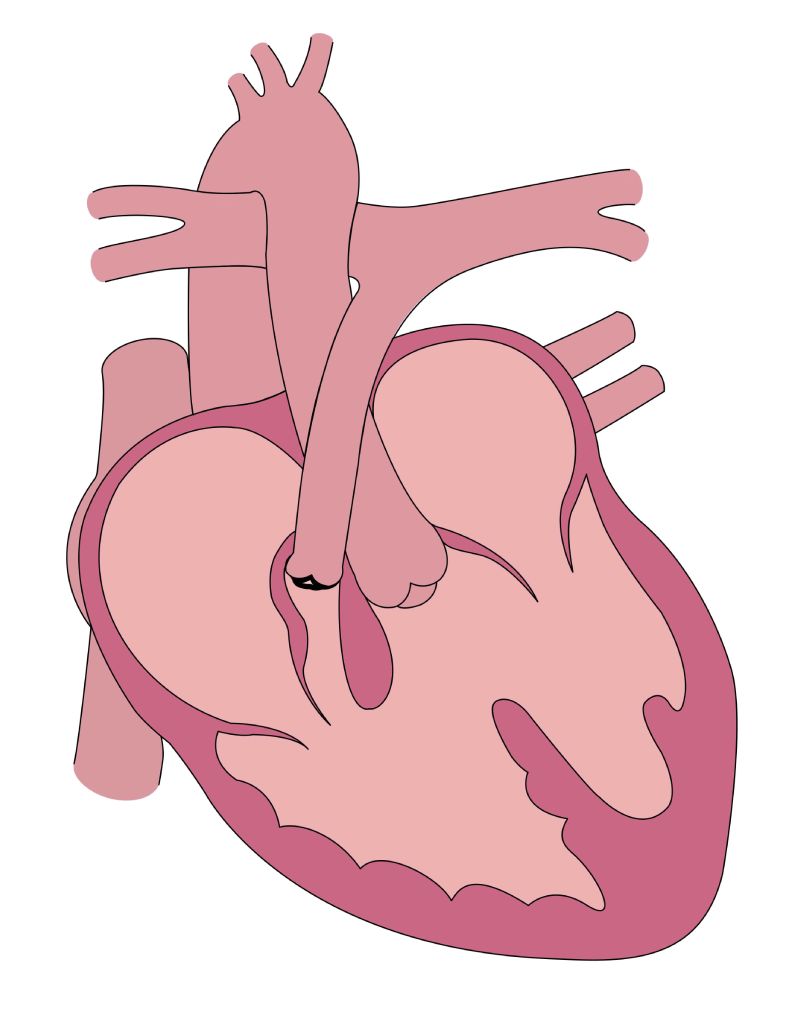
Features of tetralogy of Fallot include anterior malalignment of the conal septum creating a ventricular septal defect, pulmonary stenosis, right ventricular hypertrophy, and a misplaced aorta. (Image courtesy of Emily Harris)
Regenerative therapies for tetralogy of Fallot could benefit children with other forms of congenital heart disease, such as those born with single ventricle disease. They experience a high risk of heart failure and death as well as a need for heart transplantation. But the pool of infant donor hearts is extremely small. "These infants are the ones for whom the early benefits of heart regeneration therapies would be greatest," asserted Dr. Kühn.
If we can help infants live another year or two so they can become better candidates for heart transplantation, we will have done something good for them.
— Dr. Bernhard Kühn
While regenerating heart muscle has not been achieved in adults, evidence suggests that the barrier to grow new heart muscle in infants is lower. "Inducing heart regeneration in infants would not only present a lower barrier to overcome, but fill a real need," said Dr. Kühn. "If we can help them live another year or two so they can grow more and become better candidates for heart transplantation, we will have done something good for them."
A Mechanistic Clinical Trial
In a new mechanistic clinical trial funded by the National Heart, Lung, and Blood Institute, Dr. Kühn and fellow investigators are assessing the effectiveness of the beta-blocker propranolol for enhancing the proliferation of cardiomyocytes and normalizing heart growth in patients with tetralogy of Fallot prior to surgery.
Prior studies have shown that people without heart disease generate new cardiomyocytes into their 20s, and that this growth is reduced in patients with congenital heart disease. In tetralogy of Fallot, hyperactive β-adrenergic receptor signaling suppresses cardiomyocyte proliferation. Dr. Kühn discovered that propranolol shows promise for promoting cardiomyocyte regeneration in tetralogy of Fallot lab models by tamping down this signaling pathway.
He and his study colleagues are now enrolling infants under 45 days old with tetralogy of Fallot and pulmonary stenosis or double-outlet right ventricle disease who have not yet had heart surgery to repair the defect. They will be randomly assigned to receive propranolol or placebo. Parents give the infant propranolol or placebo in an oral solution up until the night before surgery. Patients undergo baseline echocardiography and MRI before the intervention as well as just before surgery. Surgeons typically resect muscle from the right ventricle as part of the repair. This provides a valuable sample for analysis with mass spectrometry.
Dr. Kühn's study team developed a clinical research technique using thymidine tagged with a stable isotope (15N-thymidine). When cardiomyocytes multiply, they incorporate 15N-thymidine into their DNA. Using mass spectrometry, the researchers can visualize this process and quantify cardiomyocyte division. Parents give the infant oral 15N-thymidine once a day for five days.
The parents collect urine samples from the baby's diapers for submission to the study investigators to monitor how the labeled thymidine is processed in the body. Dr. Kühn and his fellow investigators validated this approach and the use of 15N-thymidine as a marker of cellular proliferation in prior studies. "I cannot say enough good things about the parents and caregivers who participated," he said. "We asked them to collect diapers because the thymidine is excreted in the urine and babies urinate up to once an hour. Their help allowed us to plot excretion curves.”
"We hope that patients who receive propranolol will have less right ventricular dilatation, less right ventricular remodeling, and a reduced need for reoperation, such as pulmonary valve replacement," added Dr. Kühn. "We may not be able to determine these outcomes for up to a decade later."
Leadership in Pediatric Heart Care
Dr. Kühn came to NewYork-Presbyterian from the University of Pittsburgh School of Medicine because of its leadership in pediatric cardiac care, the inter-campus collaboration between pediatric cardiologists and heart surgeons at NewYork-Presbyterian Komansky Children's Hospital and NewYork-Presbyterian Morgan Stanley Children's Hospital, and the large numbers of cardiac patients available for clinical trials. "Pediatric cardiology is a collaboration, and there has been collaboration with cardiac surgery from the very beginning," he noted. "Today, most babies born with congenital heart disease survive."
Pediatric cardiology is a collaboration, and there has been collaboration with cardiac surgery from the very beginning. Today, most babies born with congenital heart disease survive.
— Dr. Bernhard Kühn
Recruitment for the tetralogy of Fallot clinical trial is underway. "We want to bring this to the attention of all pediatric cardiologists and cardiac surgeons, as well as families and patient family organizations, to let them know that we are enrolling for this trial," Dr. Kühn emphasized. "You want the best for your child now and in the future, so please come to us. There is a potential benefit for your baby in participating and a certain benefit for our field moving forward."



